This episode examines the ketogenic diet as a tool to fight against cancer. It is a follow up of the episodes on ketosis and fasting that we have done with Dr. Thomas Seyfried in episode 16, and Gene Fine in episode 36. You definitely should check those out for context before or after you dive into this one to fill in any gaps.
We are talking to someone who has actually used ketosis by a combination of ketogenic dieting and fasting as a therapy to fight his brain tumor. Our guest has gone through a variety of extreme approaches to ensure he remains in a high state of ketosis. In his case, his life depended on it. This episode is not just for those with cancer or epilepsy, but also for those interested in the benefits of the ketogenic diet. You can take some of the tools he used to improve your own state of ketosis if you are having trouble maintaining it.
– Andrew Scarborough
I met Andrew Scarborough at a conference where he spoke about his experience with ketosis and its effect on his brain tumor. After being diagnosed with a type of malignant tumor called an Anaplastic Astrocytoma, Andrew underwent several months of unsuccessful chemo treatment. He decided to take his cancer treatment and management of his epilepsy into his own hands and to go the ketosis route. This decision was based in a small part on researching Thomas Seyfried’s work, which we will also discuss in the episode.
Fortunately, this decision has yielded very positive results for him, and his tumor has shrunk. In fact, it has disappeared from scans (seen below) and his doctors are now giving him the all clear. Andrew is now working with London-based hospitals to develop clinical trials for treating brain cancer patients using an optimized ketogenic diet.
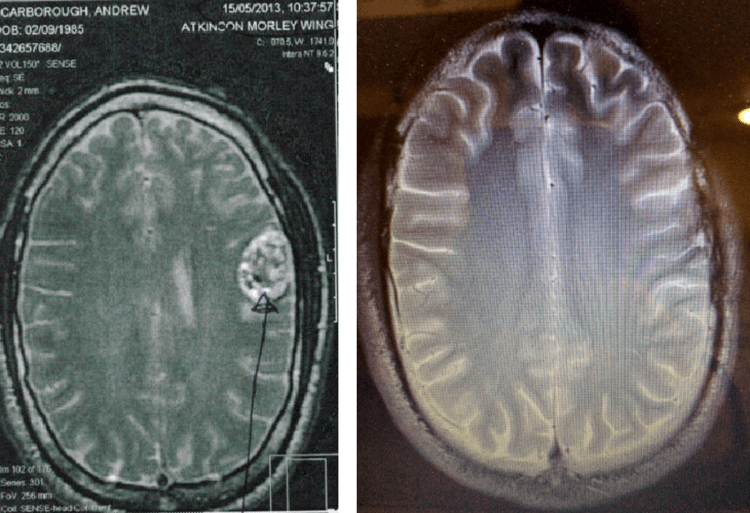
Andrew’s brain tumor before and after being on the ketogenic diet.
There are a lot of details in this podcast on how Andrew went about using the ketogenic diet, including the types of foods he ate, how he optimized the diet for his situation, the extreme measures he has taken, and how he has been able to keep up physical activity. We will talk about everything on his journey, including things like eating bugs and sheep’s brain, and quitting eating plant-based foods altogether.
The episode highlights, biomarkers, and links to the apps, devices and labs and everything else mentioned are below. Enjoy the show and let me know what you think in the comments!
What You’ll Learn
- The beginning of Andrew’s brain cancer story (4:46).
- Andrew is diagnosed with a grade 3 Anaplastic Astrocytoma (12:14).
- After unsuccessful chemo treatment, Andrew devises a treatment using the ketogenic diet (19:19).
- Using MRIs to visualize changes in the metabolic activity of the tumor due to the ketogenic diet (20:52).
- Scans show complete remission since using the ketogenic diet (23:40).
- Optimizing and maintaining the ketogenic diet for brain cancer management (26:40).
- The biomarkers Andrew tracks to monitor the effects of the ketogenic diet (28:08).
- The glucose-ketone index (29:13).
- Andrew’s typical diet (32:58).
- Maintaining a healthy 1:1 ratio of Omega-6 to Omega-3 (33:35).
- The ketogenic foods Andrew eats (36:10).
- Variations on the traditional ketogenic diet (41:30).
- Supplementing the diet with insects (46:30).
- Keeping up ketone levels and controlling seizure activity during exercise (50:16).
- Andrew’s research on an optimized ketogenic diet for brain cancer patients (54:50).
- More on Omega-6/Omega-3 ratios (59:15).
- Limiting protein and fasting (1:00:32).
- Using magnesium to prevent seizures during a fast (1:02:08).
- Mimicking chemo naturally with diet (1:06:44).
- The resources Andrew recommends for those facing cancer or epilepsy or interested in the ketogenic diet (1:11:47).
- Andrew’s advice on what biomarkers to look at and where to start with the ketogenic diet (1:18:34).
Thank Andrew Scarborough on Twitter for this interview.
Click Here to let him know you enjoyed the show!
Andrew Scarborough
- My Brain Cancer Story: Andrew’s blog.
- Andrew’s Twitter Account
- Andrew’s YouTube Channel
- New Scientist “Ketogenic diet’s reputed anticancer credentials put to test” featuring Andrew.
Tools & Tactics
Interventions
- Hyperbaric Oxygen Therapy (HBOT): A therapy Dr. Seyfried believes may be beneficial to fight cancer but is relatively non-toxic in comparison to current treatment modalities (chemo and immuno-therapies). It exposes the body to higher levels of oxygen via having the person sit in a pressurized tank with higher oxygen concentrations. Andrew is adding this therapy to his current tools. Typically you visit centers that provide sessions inside hyperbaric oxygen tanks, however some new smaller and lower pressure HBOTs are now beginning to appear in the market that you can buy to use at home.
Supplementation
- Ketosports KetoForce: KetoForce contains the endogenous ketone body beta-hydroxybutyrate (BHB) in sodium and potassium salt form. The compound BHB can be used as an energy source by the brain when blood glucose is low. Ingesting KetoForce raises the levels of blood ketones for 2.5-3.0 hours after ingestion. (Note: A similar product from the same company is Ketosports KetoCaNa). Andrew uses KetoForce to increase his ketone levels during gentle exercise.
- Ancient Minerals Magnesium Spray: Most people with epilepsy have a magnesium deficiency. Magnesium supplementation has been used to reduce seizure activity in people with epilepsy. Andrew prepares his own magnesium chloride solution that he takes transdermally multiple times every day (about 230 mg per day) and during exercise, which can be a seizure trigger for him.
- Curcumin BCM95: Curcumin is a derivative of turmeric which is an anti-inflammatory antioxidant and potentially has anti-cancer properties. Andrew takes Curcumin in tablet form with DHA because it increases the uptake of DHA to the brain.
Diet & Nutrition
- Ketogenic Diets: The ketogenic diet is a low carb diet which raises the level of ketone bodies in the blood. Tumor cells are inefficient at processing ketone bodies for energy. The diet is commonly used to help control epilepsy in children.
- Paleo Diet: A diet that mimics the nutrition of early hunter-gatherers, and consists of all lean meats and fish, fresh fruits, and non starchy vegetables.
- Water Fasts: A water-only fast of at least 3 days and preferably 5 days is recommended by Dr. Seyfried as a tool to reduce cancer risk and to lower your glucose – ketone index to 1.0. They are the standard fast protocol used in most of the research studies looking at cancer inhibition or therapy for cancer patients. Learn more from Damien’s experience with a 5-day-water-fast.
Tracking
Biomarkers
- Blood Glucose: A measure of the level of glucose in the blood at one point in time. Blood glucose is a biomarker for increased cancer risk. Therapies target reduction of blood glucose levels to limit cancer cell growth. Blood glucose levels vary throughout the day. Ideally levels should be kept below 100 mg/dL and below ~85mg/dL for fasting glucose. Andrew maintains his around 60-70 mg/dL.
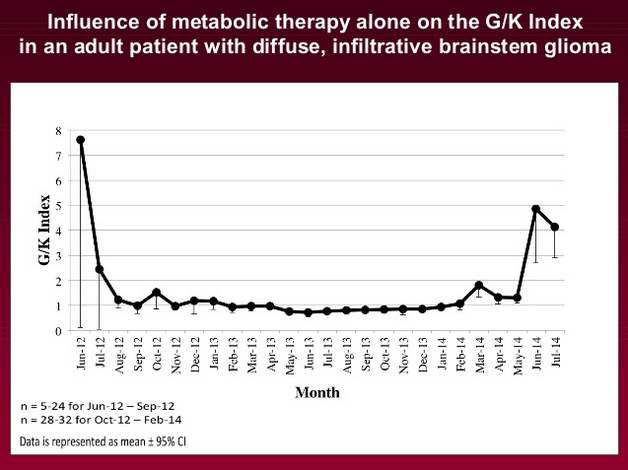
- Glucose – Ketone Index (GKI): The ratio between the concentration of glucose in the blood to ketone bodies in the blood. The calculation is Glucose (mmol)/ Ketone (mmol). Dr. Seyfried created the index as a better way to assess metabolic status. Therapeutic efficacy is considered best with index values approaching 1.0 or below. Patients with chronic disease like cancer have index values of 50 or more. Check out the episode with Thomas Seyfried here.
- Cholesterol-HDL and LDL: The cholesterol biomarkers include lipoproteins and triglycerides which are found in the blood. There are standard markers that all doctors and labs will run, and some newer specialist labs that are more specific and accurate. There are two main types of lipoproteins, HDL and LDL. We covered these markers extensively in episode 7.
- Omega-6/Omega-3 Ratio: Many Western diets are deficient in Omega-3 fatty acids, such as DHA, and have excess Omega-6 fatty acids. A high Omega-6/Omega-3 ratio promotes inflammation and the pathogenesis of many diseases, including cancer, whereas increased levels of Omega-3 (a low Omega-6/Omega-3 ratio of about 1) exert suppressive effects.
- hs-CRP (high sensitivity C-reactive Protein): a marker for systematic inflammation that can be measured over a period of time to determine effectiveness of treatments such as the ketogenic diet. Ideally CRP levels should be <1 mg/L. High levels are associated with chronic inflammation, which is common in cancer and other chronic diseases.
Lab Tests, Devices and Apps
- Glucometer: is a device used to measure the level of glucose in the blood. Andrew and Damien use the Freestyle Optium Neo Glucose/ Ketone meter. Andrew’s ketones and blood glucose levels hover around 65 mg/dl, which puts him somewhere around 0.6-0.8 on the Seyfried index. Check out episode 16 to learn more about the Seyfried Index.
- Omega Blood Count: Measures the levels of Omega-6 and Omega-3 fatty acids in your blood. (Note: This test is only purchasable via offline retail stores such as pharmacies and health shops in the UK – an alternative test that Andrew recommends that you can buy online in US or UK is OmegaQuant.com)
- Complete Lipid Panel: measures total cholesterol, triglyceride levels, HDL and LDL cholesterol, which are all found in the blood. High blood lipoprotein levels are associated with cancer.
- Complete Blood Count: is a blood panel that measures the levels of the different cells in the blood. Numbers of the different types of cells vary depending on disease status and even between people. The test is often used to monitor cancer progression and treatment.
- Magnetic Resonance Imaging (MRI): MRI scans use pulses of magnetic energy to visualize internal organs and structures. It can be used on almost any area of the body and provides information on tumors, bleeding, injuries, blood vessels, or infection. MRIs were used to monitor Andrew’s brain tumor.
- Positron Emission Tomography (PET) scan: A PET scan is a functional imaging technique used to image body processes. A PET scan can be used to identify cancer presence and severity. A radioactive tracer, fluorodeoxyglucose, is used to tag cancerous cells so they can be visualized. Check out episode 36: Quantifying Cancer and Reexamining Which Cancers May be Inhibited by Fasts with Gene Fine to learn more about PET scans and cancer.
Other People, Books & Resources
People
- Dr. Thomas N. Seyfried, PhD: University of Illinois, Urbana-Champaign. Dr. Seyfried’s research focuses on the mechanisms by which metabolic therapies manage chronic diseases like cancer, epilepsy, and neurodegenerative lipid storage dysfunctions. Check out Dr. Seyfried’s episode on “Water Fasts as Potential Tactic to Beat Cancer.”
- Dr. Dominic D’Agostino, PhD: Assistant Professor in the Department of Molecular Pharmacology and Physiology at the University of South Florida Morsani College of Medicine, and a Senior Research Scientist at the Institute of Human and Machine Cognition. His research focuses on developing and testing nutritional and metabolic therapies for neurological disorders and cancer. His own website is Keto Nutrition
- Dr. Colin Champ, MD: A board-certified radiation oncologist and Assistant Professor at the University of Pittsburgh Cancer Institute and University of Pittsburgh Medical Center. He is also board-certified in integrative medicine by the American Board of Integrative and Holistic Medicine. His focus is the role and effect diet and nutrition may have in cancer treatment.
- Dr. Adrienne Scheck, PhD: An Associate Professor of Neurobiology at Barrow Neurological Institute. Her expertise is in neuro-oncology and her lab has been involved in investigating the effects of the ketogenic diet on brain cancer.
Organizations
- Matthew’s Friends: a UK charity specializing in medical ketogenic therapies.
- Charlie Foundation for Ketogenic Therapies: a US charity that provides information about diet therapies for people with epilepsy, other neurological disorders, and select cancers.
Books
- Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer: This book, by Thomas Seyfried, is about the origins of cancer, including the theory that all cancer is a disease of energy metabolism, and provides solutions to cancer management and prevention.
- Keto Clarity: Your Definitive Guide to the Benefits of a Low-Carb, High-Fat Diet: In this book, Jimmy Moore explains the the powerful therapeutic effects of a ketogenic diet on a range of diseases, including cancer.
Other
- Ketogenic Diet Resource: Andrew says this website has answers to just about all the questions you could have.
- Clinicaltrials.gov: This site can provide you with information on clinical trials that are currently being done relating to the ketogenic diet and different cancers.
Full Interview Transcript
[Damien Blenkinsopp]: Andrew, welcome. Thank you so much for coming on the show.
[Andrew Scarborough]: Thank you for having me.
(04:39) [Damien Blenkinsopp]: Yes. You have quite an amazing story that a lot of people are very interested in hearing about. It’s always good to get the context of how this happened to you, and where it all started? Could you go into the beginning, how you made the discovery that you had this condition? How did it start?
[Andrew Scarborough]: Yes. I was studying a Master’s in Nutritional Therapy at the University of Westminster. This is before my diagnosis, and I was suffering from migraine headaches for a few months. Until suddenly I had lost my speech in February 2013, this was nearly 3 years ago now.
What I didn’t know at the time, that was my first partial seizure, and just being a man I carried on.
[Damien Blenkinsopp]: So to describe that, did you have difficulty saying words, or what exactly happened?
[Andrew Scarborough]: I went very dizzy, and then lost my speech completely for about five to six minutes, I was with a friend and we laughed about it because it was a bit strange. Because it was quite a cold day, it was February, I was just thinking when you get cold and shivering. You just stutter and loose — you struggle to speak, but it was a lot more serious than that.
I didn’t do anything about it. A couple of months later, I was experiencing very similar symptoms with pins and needles in my tongue and throat. To cut a long story short, I went on the train after a heavy gym workout. And, I felt like I actually have a lot of energy after the workout, even though I really struggled through it.
I just felt completely wiped out, even though it wasn’t the most difficult workout. I suffered more seizure activity afterwards, when I was getting on the train, very busy train actually in London to go home. And I devastatingly had a crushing headache, like my head was in a nutcracker.
The pressure was constantly building up, then I suffered a quite a traumatic brain hemorrhage, and grand mal seizure on the train, which wasn’t too pleasant, and the whole train stopped. I was rushed to hospital. There was so much blood in my brain that they didn’t know what to say, what actually was the cause.
As I was in hospital not knowing — feeling very confused not able to speak or walk at this point. I was given a CT scan and all that was shown was this massive blood in my brain. It looked like an explosion had gone off. I was still experiencing horrific grand mal seizures at this time, so I had things explained to me, and at the time, they were going in one ear and out the other, because I was so out of it.
That was quite a tough time from my family, and my first diagnosis was an AVM, which is an arteriovenous malformation. Because it looks so poor on the scans — because CT scans are quite ambiguous. All we could really see was just a tangle of blood vessels and arteries.
[Damien Blenkinsopp]: So, they thought it was an artery that had grown the wrong way, or you’d been born . . .
[Andrew Scarborough]: They saw it as being an unusual tangle of mess.
[Damien Blenkinsopp]: Okay, the arteries growing in the wrong way.
[Andrew Scarborough]: Yeah. They said, “No it’s not probably like that, it’s probably a Cavernous Hemangioma instead, which is a tangle of abnormal blood vessels, not tangled in the arteries.” Which is better because it was a bit less life-threatening, but I was given a number of misdiagnoses before. Eventually, I had an operation, because I was continually having these grand mal seizures that were starting to cause me cognitive difficulties, and my speech was getting worse, so I wasn’t able to speak at all at this stage.
(09:11) [Damien Blenkinsopp]: So, going back to the hemorrhage is that a stroke, is it the same as a stroke, or is it slightly different?
[Andrew Scarborough]: It’s very similar to a stroke, it was caused by the pressure of the tumor. Pushing against the side of my skull, and also it was between the speech movement area invading into the motor cortex, that’s why I had lost my speech completely. I had an operation not long after, in May 2013, to try and remove as much as possible, if this very vascular and invasive tumor, which was slightly larger than a size of a golf ball — but invading into the motor cortex area of my brain.
They couldn’t remove all of it because otherwise I would be completely paralyzed or dead. Because I was misdiagnosed, I should’ve had the operation awake but I was unconscious during it. The neurosurgeons said after, “Yeah we probably.”
If he has to do it again, he would have it awake so he could potentially get more out of it, but he couldn’t remove all of it because of where it was in the brain.
[Damien Blenkinsopp]: That’s interesting, what is the difference between you being unconscious and awake, are they able to get some feedback from you?
[Andrew Scarborough]: Yeah. You’re kept awake so they can monitor your responses, while they’re poking around in there to see what can be removed and what can’t, and what healthy brain tissue and what isn’t. One of the main issues with the brain surgery is it’s very difficult to distinguish what’s healthy tissue, and what’s the tumor.
[Damien Blenkinsopp]: So, this is what date now that you’ve had your surgery, and you’ve been given a clear diagnosis?
[Andrew Scarborough]: This point now? It’s two and a half years coming up to three.
[Damien Blenkinsopp]: Okay, it was a few months after your hemorrhage.
[Andrew Scarborough]: That was two months after that I’ve had the operation because they didn’t know what to do with me. There was a lot of blood in my brain, and if you think about a malignant brain tumor, it’s not a great thing if you’ve got a constant blood supply there — and it’s not a fantastic thing if you’ve had this thing that looks like an explosion in the brain, scattering around the cells, and blood everywhere. So, it just makes it more migratory, I guess if that’s the word.
More likely to spread into other areas, which is not ideal. I then had my pathology, finally, and it showed that the tumor was indeed extremely vascular. And there was still some significant scar tissue, as well as some slight enhancement there, but we didn’t know exactly what that was.
[Andrew Scarborough]: So you’re saying, is that a scan?
[Andrew Scarborough]: Yes, sorry.
[Damien Blenkinsopp]: Okay.
[Andrew Scarborough]: — This was the MRI scan after my operation.
[Damien Blenkinsopp]: Is that a straight MRI?
[Andrew Scarborough]: Yes, this was just a standard MRI, but I also had my pathology report from the amount of tumor that was able to be removed, and that came back as an Anaplastic Astrocytoma, which is a Grade 3 Astrocytoma — affecting the glial cells, the astrocytes in the brain, and quite important components of the brain. It’s not a great thing to have, particularly a high grade glioma, which is what mine was.
Brain tumors come in different gradings, so it’s like we’re staging how — with the brain it’s Grades 3 and 4 are highly malignant, and Grades 1 and 2 are slow growing. Grade 1 is typically a solid mass, that you can — if you can operate it can be curable. Even Grade 2s are known to come back, and do grow, but grow at a slower rate. But Grade 3 and 4 are the fastest growing, they grow quite fast. Mine was showing to be heterogeneous, it had quite a few Grade 3 cells in there.
[Damien Blenkinsopp]: Does that mean that it has different types of cancer cells there when you say heterogeneous?
[Andrew Scarborough]: Well, yeah. It showed numerous mutations. It’s very difficult to explain, but it showed that it wouldn’t be chemosensitive, it was negative for IDH1 which is a predictor of longest survival and chemosensitivity. It was also unmethylated for MGMT, which is a repair gene.
And that’s also — it’s not a good thing that it was unmethylated, so it was one of these gene mutations that they say is good to have for longer term survival. I also had tumor suppressor genes missing which again, with these Grade 3 tumors the timescale for survival is variable until it comes back. But in my case, I had just about the worse. It’s scenario terms with the pathology.
(14:33) [Damien Blenkinsopp]: So, did they give you a rough timeline, I guess at that point?
[Andrew Scarborough]: They said it was difficult to tell because of my age and the location of the tumor. Typically in that scenario, it’s around two years when it comes back, and that’s one of the best cases in that particular scenario. It’s a strange type of tumor because in a different scenario with different kind of pathology it can be up to five years or sometimes seven that it comes back.
It’s quite variable, but in my case it didn’t look so good, and I still had some scar tissue where there was lots of — healthy blood supply that could’ve had any enhancement that was present at the time, not great.
[Damien Blenkinsopp]: Must have been a shock, must have been a pretty big shock for you when that one came about.
[Andrew Scarborough]: Yeah, most definitely. I was told that even though my tumor was not chemosensitive that I should probably go ahead and have chemotherapy and radiotherapy, which I did for a short period because I was quite ignorant about it. I thought that it would potentially give me a bit more time.
But then once I’d looked into it I realized that it was only going to cause further mutations for me personally, and I didn’t want to see that. I started to learn my carbohydrate intake and go on a restrictive ketogenic diet after I’ve learned about it prior to my diagnosis, when I was studying a Master’s in Nutritional Therapy.
(16:17) [Damien Blenkinsopp]: Right, what was your lifestyle like before this all happened to you, and how old were you when this happened?
[Andrew Scarborough]: 27, 28. It’s difficult now thinking back, because my birthday’s at September 1, so I was 27 going on 28. It was two and half years ago and I’m 30 now.
[Damien Blenkinsopp]: So roughly 28 or 27.
[Andrew Scarborough]: Yeah. I was on a diet that I thought was healthy, so I was on a low fat, high carb with a complex carb diet, all whole foods, so I thought I was doing a good job, no processed food. I actually had quite a low body fat percentage and quite a high lean body mass. I thought I was very healthy, and I was very athletic.
I’d worked as a personal trainer for a few years. I was studying my Master’s in Nutritional Therapy and it was a shock to me that what I was learning in my undergraduate degree in Nutrition was completely useless, because I was learning all these new information that contradicted all the older information, but I was just learning about it. I thought it was interesting but it seemed to go against most of what I’ve studied for the past few years before that.
I thought I was healthy.
(17:44) [Damien Blenkinsopp]: When they gave you the diagnosis for the cancer —people at home are probably thinking, “Well is this one of those — metastasized, so it would spread to other parts of the body, or does it tend to stay concentrated?”
[Andrew Scarborough]: Yeah, well primary brain tumors typically just spread into the brain, which isn’t great because your brain is very useful. Apart from medulloblastoma, which can spread down the spinal fluid and into the central nervous system. It’s the central nervous system that can spread down the spine, and other also spread into the brain.
Mine is an astrocytoma, it would’ve just spread into the brain, and there can also be secondary tumors that come about as a response in the brain. It’s not a great type of tumor to have.
[Damien Blenkinsopp]: No, tumors are good ones to have, but it’s one of the nastier ones.
[Andrew Scarborough]: It’s the step down from glioblastoma, which is the most common type of brain cancer.
[Damien Blenkinsopp]: That always the worst, is the Type 4. . .
[Andrew Scarborough]: Yeah. I thought with my approach, with my own treatment strategy — I thought I have a little bit more time to play around with things and adjust to strict ketogenic diet. If I had a glioblastoma I would’ve pushed things a lot quicker. I did push things quite a lot, and I go to extremes with this diet and this approach.
(19:19) [Damien Blenkinsopp]: Yeah. Did you consider any other options? You said you took a little bit of chemo and radiotherapy —radiation, and pretty quickly you stopped, was that a couple of months?
[Andrew Scarborough]: I stopped after four months because I was proposed to have it for up to two years which is a long time, and I said no after a few months experiencing how horrible that was, and still having these horrible seizures. I thought, “Well, I want my quality of life to be good at least.” I stopped it, because my scans were still showing this enhancement.
I thought, “Well, we don’t know if that’s necrotic tissue or scar tissue, or if it’s the tumor activity.” But I thought that, because my tumor looked so glowing on the scan that it was potentially very responsive to carbohydrate restriction. So you do get some cancers that seem to use more glucose for energy, and you get some that actually use glutamine more for energy than glucose.
More or less they use both for energy, but because mine was so glowing up — lighting up like a Christmas tree I’d like to say, it showed that it was potentially more efficacious to just really cut down on the glucose, and see what was going to happen from that.
[Damien Blenkinsopp]: So these were all MRIs they were giving you?
[Andrew Scarborough]: Yeah, and interestingly even though it’s different from other cancers where you get a PET scan, and you can still see the enhancement there, on an MRI, that was interesting to me.
[Damien Blenkinsopp]: Do you know why that was? We spoke recently to Gene Fine who is talking about the PET scan, in the use of cancers. Do you know why you were able to see it quite clearly on the MRI in your case? Is that specific to brain cancers?
[Andrew Scarborough]: Yeah, I think from what I’ve seen in the literature it is, I don’t know exactly why that is. I guess it’s just you’re able to see the metabolic activity even with — I think it’s an iodine solution, not the good kind, the more radioactive iodine that they give you, rather than the supplemental iodine which you can get which is actually really good for hormonal control and certain cancers.
[Damien Blenkinsopp]: So, they give you an IV of that when you go to your MRI, so they can see more?
[Andrew Scarborough]: Yeah, that’s the contrast injection that they give you. Sometimes with PET scans, they do give you the — that shows up quite nicely with the contrast dye. I view my scan straight after I have them, so it’s interesting to view that.
[Damien Blenkinsopp]: Yeah. So I think its gadolinium, is that the contrast dye you’re talking about?
[Andrew Scarborough]: That’s one of them, but I don’t have that one from my scan, I have something else. I can’t remember exactly what it’s called, but I’ve had a few different kinds of scans. I’ve also had MRI spectroscopy which is a fascinating type of scan.
It works with lights, allowing you to see the microenvironment in the brain. And we’re looking at how the ketogenic diet is changing that environment within those biomarkers within the brain as I’m progressing. That’s really interesting to see.
(23:02) [Damien Blenkinsopp]: Yeah, so great. What kind of scans have you been having over time, and how frequently? And how have you seen the ketogenic diet impact that over time?
[Andrew Scarborough]: Well initially I had a standard MRI scans which were quite boring. The cancer cells, [unclear 23:19] was that wasn’t the best for brain cancer, even though it’s world-renowned for other cancers. At that time, I had the enhancement and significant scar tissue, and I had Hemosiderin, which is a blood staining, that was quite a lot of that showing on my scan.
Since then I’ve had progression in a way that I’ve been given a statement saying that I have a response, that I’ve achieved complete remission, and the enhancement is no longer present. I’ve also had significant healing of the scar tissue, and I’ve had vast improvement of my symptoms. So, I am completely off medication for epilepsy which I was told by five different neurologists — that I’d be crazy to even reduce the medication, and I should increase it because my seizure activity was so bad.
I’ve just had a linear progression of improvement in that respect, so I’m completely off medication for the epilepsy, and for that, I do a number of things which controls my seizure activity. And if I forget to do those things I instantly have seizures — it’s like being on a tightrope you have to keep up with doing all these things, I haven’t had a seizure in a long time. When I start to stop doing these things, or I slip up even a little bit I get an aura, which is a warning for me that I’m going to have a seizure.
I have emergency measures to reverse that, which I’ve devised myself largely. It’s interesting.
(25:07) [Damien Blenkinsopp]: Yeah, sounds very interesting, we’ll jump into that. So the epilepsy is a symptom, it’s driven by the hemorrhage that you had and some damage?
[Andrew Scarborough]: Yeah, and also it can provide these for an indicator of where you are with cancer with the brain. Particular with the temporal lobe epilepsy which is a typical response from a temporal lobe brain tumor. My tumor was between the temporal and frontal lobe, so I have three different types of seizures, which is fun.
Monitoring my symptoms and my seizure triggers, and my theories on what would resolve the seizures, not just the ketogenic diet but things I could do with the ketogenic diet to optimize it specifically for brain cancer management. I was able to work out what worked out most effectively for me personally and relate that to the literature as well. I was then able to go to my neurologist and say, “Well what do you think of this?”. And then when they said, “I think it’s absolutely ridiculous, there’re no science behind it.”
I was able to show the science behind it and my results. And then they could say, “Well that’s very interesting.” I’ve had success that they didn’t expect.
(26:42) [Damien Blenkinsopp]: That’s great. So when were you given the sign off, when they say, “Okay your scans are clear.” Did they say it’s in remission or do they say it’s clear?
[Andrew Scarborough]: With that kind of cancer it’s never deemed as curable and I don’t think it can be curable, but personally I think you can achieve and maintain complete remission, and maintain that status indefinitely. From close observation of the animal studies, when they come off the diet after they’ve achieved complete remission, same kind of cancers, that it comes back almost instantaneously. The unpublished human studies I know the same thing, the same occurrence.
I am very keen to stay on this very strict ketogenic diet, and I actually feel quite good on this. Internally, when I have my blood tests which I have a myriad of different blood tests just to see how I’m doing in terms of my general health. A number of markers for potential tumor progression. Internally I am actually much healthier than before I had cancer, which I find that kind of funny.
(28:08)[Damien Blenkinsopp]: So what kind of improvements have you seen, what are the biomarkers that stand out for you, the test results that have come back, and been useful?
[Andrew Scarborough]: The first thing I looked at was my vitamin D. When I was first diagnosed it was in a severely deficient range, and now it’s in the suboptimal range. People would say it’s too high now, it’s 200, and previously was 20.
I also have my triglycerides tested, I have my cholesterol done, and all those fun markers. I have a full blood count, my white blood cell count was pretty good, I can’t remember the exact figures. It’s actually better than before I had cancer, which is not typical even years after you had cancer, immunity can be compromised, so your white blood cell count is typically quite low, and I found that quite interesting.
(29:13) [Damien Blenkinsopp]: It’s great to hear about that progression. Let’s talk about the actual things that you’ve done in terms of where you started in your ketogenic diet, because I know that people said they’re ketogenic. Have you been tracking your blood ketones and blood glucose since the start? And have you seen how that’s changed as you’ve changed your diet?
[Andrew Scarborough]: Yeah. The first thing I did I went out and got a glucometer to measure my blood ketones and blood glucose, and I was comparing that to book cancerous [unclear 29:45] disease, and the glucose-ketone index that Thomas Seyfried devised and came up with, with his colleagues. I had a number of conversations with him about it, just over email, and I was amazed that he got back to me.
I found it very interesting, I started with trying to do the fast, to start with, to get me in ketosis quite quickly. But I realized with epilepsy that’s not a great idea. I had quite a few bad breakthrough seizures attempting that.
I decided not to try it that way, I decided to do it gradually and over time I managed to get into the therapeutic range within just a few weeks.
[Damien Blenkinsopp]: When you say therapeutic range what is that?
[Andrew Scarborough]: I was using the glucose-ketone index, which you use a ratio where you divide your blood ketones by the blood glucose, and you come up with a number, and you try and make sure that number is — I think it’s above one. I don’t measure it anymore in that way because I’m consistently in very deep ketosis with very low blood glucose, so I don’t have to do it anymore.
[Damien Blenkinsopp]: Yeah, we actually covered the index with Thomas Seyfried before. I think it’s a glucose divided by ketones, and there’s a couple of other little things you have to do in there, it’s not super straight forward. I put a spreadsheet up for some people who are asking, when he was talking to us he said it was under one.
So I guess that’s what you are aiming for and you seem to be saying you’ve gone…
[Andrew Scarborough]: Yeah at that time, that’s what I was aiming for, but now I’m consistently above 3.5, so I don’t have to worry about that so much.
[Damien Blenkinsopp]: Oh, in the glucose-ketone index?
[Andrew Scarborough]: Well my ketones are typically above 3.5, and the blood glucose is typically hovering around 3.5 — at the very least one to one.
[Damien Blenkinsopp]: Okay, so for the people at home, because in the US the blood glucose measurement isn’t millimolar. So you’re talking around in between 54 and 72 mg/dl, like 3-4 millimolar. I’m guessing you’re hovering around with the Seyfried Index somewhere around 0.6, 0.8.
So it’s well below one that’s what you’re saying because your ketones are so high.
[Andrew Scarborough]: Yeah. In the evenings it goes sky high, well the ketones go sky high, the glucose goes really low.
[Damien Blenkinsopp]: Do you mean from 5 o’clock onwards — it’s interesting because I saw that in some of my fast and some of my earlier experiments also.
[Andrew Scarborough]: Yeah. I guess it’s a hormonal thing that happens, and also because there’s that period of time where I only have typically two meals a day, that’s the in-between period, I guess where it goes that high. So that’s where I’ve unintentionally fasted for that period of time even though the diet’s mimicking fasting itself.
(32:58) [Damien Blenkinsopp]: What is a typical day look? What are you doing now, what is your typical day look like? I’m assuming at the moment you’ve got the most extreme version of your own program for this, is that correct?
[Andrew Scarborough]: Yeah. Typically I have 85% of fat and 15% protein in my diet, but over the last few days, I’ve experimented with 90% fat and 10% protein, and negligible carbs. Typically on my 85% and 15% protocol that I follow which is very similar to the animal studies, and quite similar to very strict ketogenic diet for children with epilepsy.
I restrict my calorie intake to 1,600 calories — calorie restriction is extremely important for brain cancer management. You probably discussed that with other people I’m guessing. What’s also important I think is the other things that I’m doing.
Personally, I think it’s very important to make sure you have correct therapeutic ratio — I like to call it of omega 3 and 6 in the blood, and I have at home testing kit for that which I send off to the lab every few months.
[Damien Blenkinsopp]: Okay, that’s interesting, is that a dry spot test?
[Andrew Scarborough]: Yeah, it is. You just have to collect quite a significant amount of blood, and it gives you a report back just saying what you’re ratios of omega 3 and 6 are in your blood.
[Damien Blenkinsopp]: Which lab are you using for that?
[Andrew Scarborough]: Well, the testing kit is by — if you go on Omegasense.com it comes up. There’s a center called the NutriCentre in London, and I just get it from there. It’s a pretty good test, very accurate.
[Damien Blenkinsopp]: Have you seen that change? This is actually the current levels ratio, it’s not like it’s your diet of the day like we were talking about — the blood glucose and the ketones which are changing all the time. It’s a more stable marker which is evolving over time, so you’re choosing for a range you want to keep it within.
[Andrew Scarborough]: I’m just trying to get us close to 1:1 ratio as possible, and I’ve experimented with a 2:1 and a 3:1 ratio in favor of omega 3 which is quite hard to do, but it’s very interesting. We know that omega 3 fatty acids exhibit neuroprotective properties and can represent a potential treatment for a variety of neurodegenerative diseases. It’s really interesting, we know that they are shown to be cytotoxic to tumor cells themselves.
Ideally, an optimal ketogenic diet for brain cancer should have, in my view a better ratio than omega 3 and 6. I think the standard ketogenic diets that are applied to humans at the moment are way to high in omega 6 which is inflammatory. I struggled when I was doing a standard ketogenic diet because of that.
[Damien Blenkinsopp]: What are you taking in order to raise your omega 3 levels? What are you doing in diet specifically?
[Andrew Scarborough]: Well, initially I was eating lots of brains because they are the best source of omega 3 that you could get, and that’s high in DHA, and one of the main fatty acids in the brain is DHA. The brain is 70% fat, and the rest is mostly water, it just makes sense to me to have in my diet mostly fat and water, that was my main reason for doing that.
We also know that the fatty acid composition of gliomas differs from that founding non-malignant brain tissue quite significantly. The reduction of glioma DHA content is really interesting to view — we know that in gliomas which is what my tumor was, and what a glioblastoma is as well. We know that they have significantly less DHA in and around them.
If we can increase that — the literature shows that it can have a very potent effect, particularly when on a ketogenic diet, in shrinking these tumors.
[Damien Blenkinsopp]: That’s great so you’re still eating brains today, is this a large part of your diet? What types of brains?
[Andrew Scarborough]: I was eating lamb’s brains, but, unfortunately, I’ve stopped eating them because of the very, very low risk of Scrapie which is like a CJD, a Mad Cow disease but the lamb form. Even though it’s a very small risk, and you probably have that same risk if you were to eat any infected tissue of that same animal, I just thought it would be a good idea to avoid it, which is a shame because it’s my favorite type of food on the ketogenic diet.
It’s a perfect ketogenic food, but my second most therapeutic ketogenic food that I found is sweetbreads which is the pancreas and the thymus gland of — in my case I get them from lambs again. I’ve done an experiment which is on YouTube, on my YouTube channel, just look at Andrew Scarborough, and look at my sweetbreads experiment, I’m testing the myoglobin of sweetbreads and it comes up very high on the glucometer for ketones.
When I test my blood after my postprandial blood glucose and my blood ketones after eating, my ketones shoot up very high, and the blood glucose stays more or less the same as before I started eating.
[Damien Blenkinsopp]: That’s interesting. Out of interest, how much do sweetbreads cost? Are they relatively cheap or expensive?
[Andrew Scarborough]: Well I mostly get them for free, sometimes I have to pay a pound for them.
[Damien Blenkinsopp]: Okay, so they are very cheap.
[Andrew Scarborough]: Yeah, because no one wants them.
[Damien Blenkinsopp]: Right that’s what I was thinking.
[Andrew Scarborough]: They’re incredibly nutrient dense, rich in trace minerals such as zinc and selenium, and they’re rich in protein, and omega 3 fatty acids. Like the brain, and like all the fish — the great source of omega 3. They also raise ketones very high.
[Damien Blenkinsopp]: Yeah, that’s very surprising. I don’t know if you’ve heard new supplement ranges which I’ve been playing around with it, exogenous ketones.
[Andrew Scarborough]: Yeah, I take those as well. I take KetoForce, mostly when I’m trying to do exercise because exercise is a huge seizure trigger for me. So yeah I play around with that.
[Damien Blenkinsopp]: It sounds like the sweetbreads are more effective than the KetoForce, KetoCaNa and the other ones.
[Andrew Scarborough]: Yeah. I actually made a supplement, a sludgy juice that the sweetbreads come in because I have them completely fresh straight after the animals are being slaughtered, well not straight after, but not long after, because they have to do a number of things just to make sure they are safe to eat. I made a supplement out of that and tested it, and it was very interesting the results, but it tasted absolutely foul.
[Damien Blenkinsopp]: Is that a downside of sweetbreads, they’re really awesome except they taste bad.
[Andrew Scarborough]: Yeah.
[Damien Blenkinsopp]: Okay.
[Andrew Scarborough]: It’s not the best tasting, you have to boil them for a long period of time, but they’re very nutrient dense and very effective.
[Damien Blenkinsopp]: How do you eat them? Have you got a quick recipe for the people at home, and they’re like, “Oh like a great thing to try out.” But if it tastes horrible is there some way to mask it.
[Andrew Scarborough]: The best thing to do is boil them for about an hour, that’s actually a short period of time typically for sweetbreads. Normally, it’s a lot longer. And then if you add tarragon to it, it actually compliments the flavor, and it actually tastes a lot nicer.
That’s one of the things I do, it goes well with tarragon. I just consume every bit of the animal, and I don’t have any carbohydrate so that’s how I get around possible nutrient deficiencies from not having any fruits and vegetables. And it allows me to not count carbohydrates, so it’s a Paleo-Ketogenic diet.
[Damien Blenkinsopp]: It’s a pure meat diet, right? Basically a pure carnivore?
[Andrew Scarborough]: Meat and fish, and fat, and that’s it.
(41:37) [Damien Blenkinsopp]: I do know there’s a little bit of story behind the reason — first you were on a ketogenic diet and you were doing more of a straight forward one with the coconut oil, and all of these kinds of things, what happened?
[Andrew Scarborough]: I noticed that with certain people with certain types of brain injury, your brain can be more sensitive to salicylates which are found in coconut oil, various vegetables and fruits, especially ones that have seeds. I wasn’t able to have avocados or any of the staple ketogenic foods that you have. I also couldn’t have dairy because I had a reaction to that, and I wouldn’t advise dairy anyway on a ketogenic diet for anyone with cancer let alone — brain cancer, because of IGF-1.
It just doesn’t make sense to me that there’re so many ketogenic diets for cancer management that have been based around dairy.
[Damien Blenkinsopp]: Right. There’s a lot of cheese, cheese is pushed quite hard…
[Andrew Scarborough]: Yeah, loads of cheese and double cream, and it’s not efficacious for me, even though I’m astounded that they get any results with these trans fat. And they do get some results, that’s encouraging for me on my — what I would call a more beneficial and effective ketogenic diet for this circumstance.
(43:06)[Damien Blenkinsopp]: Could you explain quickly the IGF-1, because there are people at home that are not quite up to speed on the IGF-1 and the dairy aspect of it. What’s the problem there?
[Andrew Scarborough]: It activates insulin-like growth factor and that can cause cancer cells to proliferate faster. One of the ways I get around that — I used to eat lots of butter, but because it’s more insulinogenic and it has milk proteins and casein. What I do is I have Ghee, which is clarified butter so the milk solids and the casein have been removed, and it’s much less insulinogenic and I actually get a much better blood ketone readings as a result as well compared to butter.
I find that interesting in itself, and we also know that compared to coconut oil, Ghee has much more omega 3 fatty acids, and coconut oil only has omega 6. If you’re basing a ketogenic diet around — just loads and loads of coconut oil which is just omega 6. Even though coconut oil is fantastic for achieving ketosis, I would advise it in moderate amounts if you can tolerate it because it’s really good.
I would say that making sure that you have enough omega 3 by having more animal fats is more beneficial in terms of the overall nutrient profile than just consuming tons of coconut oil.
(44:44) [Damien Blenkinsopp]: Right. You mentioned you eat all the parts of the animal, I’m guessing you mean all of the organs…
[Andrew Scarborough]: Yep.
[Damien Blenkinsopp]: Do you consume what you would call a variety of these? Do you try to cycle them, and the widest spectrum possible? So what other organs are you eating, are you literally eating all of the different organs on a rotation each week?
[Andrew Scarborough]: Yeah. Literally everything but mostly heart, because it’s very very cheap, it would cost me 60 pence at a time, and you get quite a substantial portion— because lamb hearts are quite fatty, there’s a huge chunk of fat on them. I can just eat them as they are, and I don’t need to add extra fat.
It’s a fantastic source of iron, zinc, selenium, B vitamins, folate, and it’s the best food source of coenzyme Q10. It’s funny how people pay an absolute fortune to get pills that have a coenzyme Q10, and I just get the best source that you could possibly get for 60 pence at a time.
[Damien Blenkinsopp]: There’s a psychological barrier about the taste, and it’s just what we’ve become used to really. I’m definitely nowhere near as far as you — I’ve been eating more organ meats and I’m trying to push it up, I just made another order today from a new company actually. I’m slowly building my way up, and it’s a taste I’m struggling with, recipes I think help with that, learning how to cook and deal with the different tastes, and just getting used to them.
[Andrew Scarborough]: Yeah. I actually did quite well to start with brains, they’re actually the most tolerable in terms of tastes because they just taste like creamy eggs.
[Damien Blenkinsopp]: Oh, I would’ve never thought that.
[Andrew Scarborough]: They taste like creamy salty eggs.
[Damien Blenkinsopp]: You just don’t look at them while you’re eating them.
[Andrew Scarborough]: No. And a number of things I do are just for entertainment, to keep the diet interesting, to make sure I have enough trace minerals. That’s why I added insects to my diet quite early on because anytime you eat the whole animal you’re getting a variety of nutrients. When you eat insects you’re consuming the whole animal — it just makes sense that it would be a beneficial thing to have.
[Damien Blenkinsopp]: How do you consume those? Because I know there are cricket bars out there in the US, how are you consuming insects?
[Andrew Scarborough]: What I do is I get the fattiest insects that are ketogenic, I get waxworms and super worms. Mostly insects that reptiles eat, I get them from a pet shop that sells them for reptiles now, I used to get them online.
[Damien Blenkinsopp]: Oh, man. Okay did you used to buy from [check 47:31 – Bug Grow], was that the specific brand — was that the only place you bought from?
[Andrew Scarborough]: Yeah, I tried a few, I tried silk worm, pupa as well — a few different insects have different medicinal properties, they’re in Chinese medicine. They’re really interesting in terms of the properties that they have. But we largely ignore that, mainly what I do now is I get them from the pet shop.
I just stick them in the freezer to kill them, and then I’ll give them a gentle wash and eat them …
[Damien Blenkinsopp]: You just eat them straight?
[Andrew Scarborough]: The problem, if you get them online is that they’ve been dehydrated and cooked so much that the nutrient profile isn’t as good as if you have them fresh after they’ve been wiggling about. I also grind them up and make my own flour after I’ve frozen them. That makes quite nice breads, I make a zero carb ketogenic bread which is very useful. People actually think it’s proper bread…
[Damien Blenkinsopp]: You don’t tell them right?
[Andrew Scarborough]: I’ve actually offered it to people without telling them, and they quite like it, and then I tell them what it is, and they want to punch me. But it’s actually surprisingly quite nice.
[Damien Blenkinsopp]: A quick story here, I was in Mexico 15 years ago and I went to Taxco. Anyway you go up into the mountains, into this old city and they were selling plastic bags full of live insects for eating. It’s something that we used to do — we don’t do in modern society. . .
[Andrew Scarborough]: If you look at anthropology, and how we evolved, it’s largely ignored especially with these Paleo diets — we evolved primarily eating a variety of insects, and in quite a large amount. It suggested that the man would go out and go hunting — would only about a 20% success rate catching these larger animals.
The woman would be mainly collecting insects for food. Seasonally they would collect nuts and berries, but it’s a fact in anthropological studies that we did consume a large amount of insects before we moved closer to the coast to eat fish, and that’s how our brains developed more. It’s an ignored fact.
(50:16)[Damien Blenkinsopp]: It’s really interesting, we’ll get there. There’ll be people writing books — maybe you, about the missing parts of the Paleo diet, Paleo upgraded. You did mention that, when you exercise you’re taking exogenous ketones, because of your epilepsy, why is that?
[Andrew Scarborough]: When I exercise my blood ketones go down, lower than my individual therapeutic reading for seizure control for me personally. I have to do that, and I also have to take another experimental treatment of mine which is proved effective, which I learned from the literature on epilepsy. It’s a magnesium chloride solution that I mix into water, and I have a specific amount that reverses auras.
An aura for me is when you have all symptoms that you’re about to have a more serious type of seizure. An aura is a partial seizure in itself.
[Damien Blenkinsopp]: Okay. Maybe you would loose your words a little bit?
[Andrew Scarborough]: I would get pins and needles in my mouth and throat, and I would feel very dizzy, and faint. I have this horrible feeling like I’m going to collapse and have a tonic-clonic seizure. When I take the magnesium solution that I take three times a day, it actually reverses that aura, it is a potent preventative measure that I found to control seizure activity extremely effectively.
People with any kind of epilepsy, their levels of magnesium drop very low, and there are certain types of the day that magnesium is at its lowest, and typically that’s when seizure threshold is also at its lowest. If we can control that, we can control seizures very effectively. Also, on a ketogenic diet, supplemental magnesium — particularly magnesium chloride are found most effective.
It acts as a natural statin, it has a beneficial effect not only on cholesterol, in a natural way not like a typical statin where it’s actually destroying that process, it’s working with your body to do it naturally. I find that it also controls blood glucose — it regulates blood glucose very effectively too. I see it as my replacement for my medication that I was on previously, and the medication interestingly actually causes magnesium deficiency as well as calcium deficiency, deficiency in vitamin B-12 and vitamin D.
[Damien Blenkinsopp]: Which medication where you on?
[Andrew Scarborough]: I was on the maximum dose of Levetiracetam, which the brand name is Keppra and Sodium Valproate the brand name for that is, Epilim. I was both on those and the highest possible amount that you could be on. You can imagine the side effects of that, and the nutrient deficiencies that caused were just quite substantial.
When you’re withdrawing from those drugs you could actually get breakthrough seizures if you don’t address those nutritional deficiencies, and those seizures can actually cause SUDEP — it’s shorthand for sudden unexpected death in epilepsy. I was told consistently that I was highly likely to have that if I was to — not only come off my medication which is what I eventually did but reduced the medication. I have to reduce that medication for a period of almost two years.
I had to do it very slowly, and adding these nutrients and trace elements so that I was not having these breakthrough seizures that were life-threatening. It was a difficult balance, but I achieved it.
(54:50) [Damien Blenkinsopp]: It makes it easier when you titrate down slowly, but still you’ve been courageous in pushing for all of these things when you’re getting this pushback which is saying it’s really dangerous. Just in terms of the exercise, how do you bump your ketones up – is it the KetoForce?
[Andrew Scarborough]: Yeah. I consume that throughout my workout but I tend to mostly just do quite a light bodyweight exercise because I don’t want to stress my body too much. Thomas Seyfried himself recommends that cancer patients don’t push themselves too much with exercise, because it just puts too much stress on the body and on the brain. Mostly I just go for long walks, in an area with lots of oxygen, and I’m actually going to start having hyperbaric oxygen therapy fairly soon.
I’m in discussions with a number of facilities about that, and I’m going to start doing case studies on patients. I’m actually working part-time at the moment with Imperial College London in Charing Cross Hospital, to start-up clinical trials hopefully next year with brain cancer patients using — what I would call an optimal ketogenic diet.
We’re looking at magnesium for these brain cancer patients, we’re looking at the omega 3 and 6 ratios in the blood, we’re looking at C-reactive protein as a marker for a systemic inflammation, and we’re able to measure that for over a period of time to see how that changes while on a ketogenic diet.
[Damien Blenkinsopp]: With cancer is that typically high the hs-CRP because of the inflammation, or is that just a. . .
[Andrew Scarborough]: Yeah. It’s typically higher than normal, but one of the main ideas of measuring that is to have a marker that you can measure over time. I’m a huge fan of testing and I know that even if these things have no effect on cancer, they have an effect on epilepsy and blood glucose management.
We know that these are prognostic factors and they’re also effective at managing epilepsy which many brain cancer patients have as a result. I’m very keen to start doing this in patients more, and I’m working very hard to do that.
[Damien Blenkinsopp]: It’s very exciting that you’re able to work in hospitals. This is starting next year you said, potentially?
[Andrew Scarborough]: Yes. It would also be featured in, New Scientist magazine early next year. My story and my approach will be featured, and that’s very exciting as well because it’s getting the message out there and we can then have the actual data on humans which is missing. It would be — as I’ve said before it will be efficacious.
We’ll be able to not just translate the diets that have been used for children with epilepsy which I don’t believe …
[Damien Blenkinsopp]: As good, as they could be?
[Andrew Scarborough]: I don’t think that they’re translatable for brain cancer patients because I think it’s just very different. For example, when I was on the standard type of ketogenic diet, they did include those ingredients. I developed symptoms that were similar to Temporal Arteritis, where my temporal arteries became so inflamed that I nearly went blind and I was prescribed steroids for it.
But instead of taking the steroids what I did is I looked at how much omega 6 I was taking in my diet, and even though my blood glucose and ketones looked fantastic, and the ketogenic diet is anti-inflammatory in itself. I was having these inflammatory responses which were only controlled and reversed when I re-addressed the balance of omega 3 and 6 ratios. That in itself is quite powerful.
(59:15)[Damien Blenkinsopp]: Interesting. Where did your omega 6 ratio start? We read studies where the standard American diet, for example, is you can get ratios of 20:1, 10:1 — quite far off.
[Andrew Scarborough]: I’ve read up to 40:1.
[Damien Blenkinsopp]: Were you not so bad because you said you had a reasonable — you were trying to have a reasonably healthy diet before. I wouldn’t expect you’d have the sad numbers.
[Andrew Scarborough]: Yes, prior to initiation of the diet, I would say I was most likely about a 10:1 ratio. But, on the ketogenic diet, it was probably quite similar actually because it was including lots of nuts, coconut oil, coconut milk, coconut cream, lots of vegetables that were high in omega 6. I just thought it could be done better — then I transferred on to what I like to call a, fishogenic diet.
I was consuming a lot more fish, and I felt instantly much better and then as I cut down on the vegetables – cut them out completely. I had an instant response where I can’t even remember the last time I had a headache, even a mild headache.
(60:32)[Damien Blenkinsopp]: Great to hear. I’m conscious of your time I know that you’re really busy currently. But there’re a couple of things — I do want to make sure we cover before you go. We didn’t speak about glutamine and I know that an important part you mentioned up front that’s something you had to restrict quite sharply. But how did you do that practically?
[Andrew Scarborough]: Well, the first thing I did was limit protein quite significantly, and I did a number of therapeutic fasts, and it wasn’t until then that I actually saw the greatest response in my MRI scans, in terms of the complete remission. One of the other things that’s quite effective is with the magnesium it has an effect on that as well. I need to find the study for that, but I can send it to you if you’re interested in reading it.
Another thing that I’m actually looking into for the long term is Metformin, because Metformin on a ketogenic diet has quite a potent effect. It has a number of mechanisms which I can’t remember all of them off the top of my head, but that’s one thing that I’m playing around at the moment. It gets an effect on MAMP and a few other things.
It’s quite hard to explain, it’s quite technical.
[Damien Blenkinsopp]: In terms of the fast, you said that’s when you really started seeing the effects, so that would mirror — we had Thomas Seyfried on here and he was talking about the importance of the fast. How many days — was that a pure water fast? Was it a seven or five day fast?
[Andrew Scarborough]: It’s interesting because I think that — when these researchers are talking about fasting for brain cancer patients particularly if they have epilepsy, what they fail to note is that there’s ionic changes that are happening in the brain when you’re doing these fasts. A patient with epilepsy can’t — especially if they have brain cancer in my opinion shouldn’t just do water-only fast.
I think that they need to do what I call, a ’magnesium fast’. When I fast I have my magnesium water solution that I make up myself, and that prevents me from having breakthrough seizures while I’m fasting because I have such low body fat percentage. My longest fast has only been nine days. I aimed for 10 but I couldn’t do more, I’ve done that a few times but I need to have my magnesium-chloride solution or I instantly have breakthrough seizures, not the good kind either.
I found out the hard way initially, but now it’s just the easiest thing that I do.
[Damien Blenkinsopp]: You’re taking specifically magnesium chloride, is that because it’s a spray kind or is it actually the magnesium chloride specifically — there’s something about the chloride which is helping?
[Andrew Scarborough]: It has something to do with hydrochloric acid and how you digest it. I’d say it’s more bioavailable and it seems to me to be just in my personal experiences that it seems to get the brain very quickly. The literature doesn’t actually say that, but personally, I found that — even though there is not much in the literature about that.
[Damien Blenkinsopp]: Are you buying a specific brand? We’ve talked about using magnesium spray transdermally, but I’m just wondering if you’re using one of those sprays? How much you’re taking of it?
[Andrew Scarborough]: It’s designed to be primarily used transdermally this particular type, and I just get it from a health food shop, it’s mainly people who do sports who take it, which is interesting and funny. I typically take about five sprays three times a day. I can’t remember exactly how much that is, for 10 sprays it’s 150 milligrams of magnesium.
It’s variable depending on how mixed up the solution is — typically around 230 milligrams in a day that I would take. If you consider our water is too high in calcium and not high enough in magnesium. It’s addressing that imbalance that we have, we know that we should have at least a 2:1 ratio of magnesium to calcium, that addresses that imbalance.
We know that in the mornings after we wake up, magnesium levels are lowest. Primarily take it in the morning, after waking up in the afternoon, and before I go to bed.
[Damien Blenkinsopp]: Have you checked your RBC magnesium levels?
[Andrew Scarborough]: I haven’t because I don’t think it’s an accurate measure. I just go by how I feel, and sometimes — I see the epilepsy as a blessing because everything to do with epilepsy with brain cancer is typically very similar to what would work for treating the cancer. If something is working for the epilepsy, you’ve got a pretty good idea that it’s beneficial for the cancer, and most of the things that I actually research about what helps in terms of my epilepsy, experimentally and otherwise.
I found incidentally that it has quite potent anti-cancer benefits as well. It’s really interesting the relationship. It’s quite empowering as well. What I would call spectacular results because I still can’t believe I’m not having these horrific seizures all the time without medication. It’s quite empowering to know that it’s potentially having the same benefit on the cancer.
(1:06:44)[Damien Blenkinsopp]: Yes, it’s pretty amazing your journey. I don’t know if you’ve come into contact with other people with similar stories to tell — I know that some other people who had cancer, you said, unfortunately, they’ve passed away — the ones you were relating to. But if you come across any other people who have been experimenting like yourself.
[Andrew Scarborough]: Yeah. I actually have a group of friends now who I came into contact with just through seeking out long-term survivors, and I have a group of long-term survivor friends who had glioblastoma many years ago, and now have no sign of disease. I have a group of friends with various other cancers who are still here now. They’ve mostly done a drug cocktail treatment on themselves, which is very interesting.
Personally, I wanted to try and copy that drug cocktail treatment but do it in a natural way just using diet.
[Damien Blenkinsopp]: When you say drug cocktail, is that chemo or is that more Metformin and things like that?
[Andrew Scarborough]: It’s more Metformin and statins, and phosphates, and various other DCA, and other very interesting drugs. Personally, the only one I’m considering is Metformin, and potentially a few others, but mainly Metformin and Curcumin which I take in tablet form with DHA because they work synergistically. Curcumin actually increases uptake of DHA to the brain.
Because we know that around these tumors, or where the tumor was – DHA is very low. We know that if you have Curcumin and DHA that’s a powerful combination. Curcumin is cytotoxic to the cells. We know that DHA is, and is essential for brain functioning.
[Damien Blenkinsopp]: You really have built a whole lot of armory against this — it sounds like you’re doing really well. On the Curcumin – there’s many forms available on the market today, you’re taking one of the bioavailable forms…
[Andrew Scarborough]: Yeah, it has piperine in it as well.
[Damien Blenkinsopp]: Okay.
[Andrew Scarborough]: It’s a component of black pepper. I have a number of strategies that I use, and I’m constantly optimizing my metabolic formula.
(1:09:14)[Damien Blenkinsopp]: Do you feel constant improvement? I don’t know if there are any symptoms because it seems like you’ve got most of it under control. Do you think you’re going to be able to repair your body, do you feel any signs of that in terms of potentially resolving the epilepsy?
Do you think this is more likely something that you’re just going to optimize and maintain so that it never bothers you, so you never get the actual symptoms?
[Andrew Scarborough]: As my brain has been visibly healing at a very fast rate on these scans while I’ve been utilizing this protocol, I’ve also found my symptoms have improved with that quite substantially as well. I had facial paresthesia constantly all throughout the day, everyday, and a number of other debilitating symptoms I couldn’t even go out and walk a few steps. The fatigue was horrendous as well.
Being able to do what I am now and this non-stop activity, and just doing so many different things, and having my seizure activity controlled in such a great way that’s much better than before — even before when I was doing all these things I was still getting more activity. I haven’t actually done that many more things if I compare to even just a few months ago. Definitely improving in quite a dramatic way, despite having to keep up with all these things.
It’s getting easier to control, to the point where I have days now that I have no symptoms at all, but if I get overconfident and I forget to have my magnesium drink or do something that’s just out of my routine, I’d definitely have more seizure activity coming. Even though it’s not to the degree that I used to have.
[Damien Blenkinsopp]: I guess really say why you’re saying epilepsy is a bit of a bonus for you because it’s early warning detection system for you…
[Andrew Scarborough]: Yeah.
[Damien Blenkinsopp]: — Whereas cancers can creep up on you and you won’t know unless you’re watching the scans and even the scans aren’t showing a small progression. So right now you can still have a small amount of cancer left, but you can’t see it. It does seem like a pretty nice little tool, even though it’s not nice to have it, in the longer term it sounds like it’s a beneficial thing for you.
[Andrew Scarborough]: Yeah, I can see it as beneficial now, I couldn’t before but it definitely is.
(1:11:47) [Damien Blenkinsopp]: Well Andrew this has been an amazing — it’s very inspiring episode today. I can really say that — I’m totally going to take some of the things that you have been trying and start testing them out myself. I would like to ask you — where to look first if they would like to learn about this topic if they’re facing cancer or epilepsy?
Are there good books or presentations on the subject, the first places to go to, to start learning themselves about this?
[Andrew Scarborough]: I would thoroughly recommend the book, Cancer as a Metabolic Disease by Thomas Seyfried. I think that’s a great starting point. For anyone starting a ketogenic diet I would recommend, Keto Clarity, that’s a good resource to use. I would also go to www.ketogenic-diet-resource.com — that has answers to just about all the questions that you could have.
For help to a dietician, if you live in the UK I would recommend the charity, Matthew’s Friends. In the US, I would recommend the Charlie Foundation which is the sister organization of Matthew’s Friends in the UK. It has recently started to see — it’s mainly brain cancer patients that they see because they get around with that by saying that they’re treating the epilepsy.
I would also go on Clinicaltrials.gov to see what clinical trials are happening globally to do with the ketogenic diet and different cancers.
[Damien Blenkinsopp]: Right, so if they’ll just search for a ketogenic diet on there…
[Andrew Scarborough]: Yeah, if they search for ketogenic diet and cancer on Clinicaltrials.gov they can see all of the clinical trials that are currently happening in terms of ketogenic diets for different cancers. It’s very exciting that more and more of these are popping up, and I hope to — I have a meeting on Thursday to discuss having proper official ketogenic diets, using the right approach in this country, and that’s really exciting new development.
[Damien Blenkinsopp]: Is that with the government, NHS or some other body that’s going to help promote it.
[Andrew Scarborough]: This is in conjunction with brain tumor research, they’re one of the very few cancer charities that actually are going all at it with this metabolic research, and they’re doing that with Imperial College London. It’s a small charity that’s doing this, it’s quite incredible what they are able to do being such a small organization.
[Damien Blenkinsopp]: It’s great they’re starting to be – some grounds building from the bottom and up.
[Andrew Scarborough]: Yeah, and I’m going to start-up my own individual research with a few of my lecturers at my university because I want to get these things happening much faster than if it’s going through clinical trial protocol. I want to do this myself with lower grade gliomas, so that we can see a long-term response to try and shrink these tumors hopefully, because they are not as aggressive, but, they still are incurable.
I want to see what effect that we can have on them rather than having to go through all the standard treatment to go through clinical trials. I think that’s very exciting going forward.
(1:15:25) [Damien Blenkinsopp]: That sounds really exciting, and I’m sure anyone who – maybe affected would be very interested to know more. What are the best ways for people to connect with you and learn about you, and keep up with you when you’re doing these things, they can stay up to date on them. Are you on Twitter, you mentioned you had a YouTube channel?
[Andrew Scarborough]: Yeah, my Twitter name is @ascarbs, and I’m on Facebook if people want to add me on there, Andrew Scarborough. I also am working on a website at the moment which is www.metabolictherapy.co.uk, and that has a holding page at the moment, but it should be live shortly. I have a YouTube channel, Andrew Scarborough, and I have a blog, My Brain Cancer Story that’s the title of it.
People search for Andrew Scarborough and My Brain Cancer Story, they should find it.
[Damien Blenkinsopp]: Excellent. We’ll put all those links on the show notes of course also, make sure all of that is there. Is there anyone besides yourself you’d recommend to learn more about the stuff that you mentioned, Thomas Seyfried, is there anyone else that people should look to?
[Andrew Scarborough]: I would look at the research by Dominic D’Agostino, also I would recommend Dr. Colin Champ, I’ve had various discussions with him online which are very interesting. He’s very interested in my approach and he is very unique, he’s a radiation oncologist who is very supportive of this metabolic treatment. Very similar to my oncologist who – it’s quite a rare thing to find – but it’s very encouraging.
There’s Dr. Adrienne Scheck, who I’m having a meeting with on Thursday she’s coming overseas from the Barrow Neurological Institute in the US, and she’s the one that does the rodent studies using the ketogenic diet. It’s great to be able to discuss with her.
(1:17:29) [Damien Blenkinsopp]: Great, great, thank you for those. Some quick items on your – just a personal approach on what you would advise people to get started with – are you still tracking any biomarkers, on a routine basis?
[Andrew Scarborough]: Only occasionally with MRI spectroscopy but we’ve stopped doing that now just because it looks a bit boring and nothing’s really changing. It all looks really good, that’s why we’re not monitoring it anymore.
[Damien Blenkinsopp]: So maybe once in every six months or once a year?
[Andrew Scarborough]: Yeah, just to keep an eye on it, but everything that you would expect to be elevated but would be a bad thing isn’t showing up – it sounds like a good thing. It’s very new research, we don’t know too much about it, but it’s very promising for the future.
Because if we can see these things before they show on the scan, in terms of enhancement or just showing in an obvious way then it’s – that can only be good for the patient really. Then we can intervene in a non-toxic way.
[Damien Blenkinsopp]: So if you were to recommend one experiment, basically you’ve done many experiments to get to this point – they’re not proven recommendations by doctors and so on. What would you recommend that someone with brain cancer or potential other cancer – what would be the first thing they should try, the biggest payoff from all of the things that you’ve mentioned, what should their first step be?
[Andrew Scarborough]: The first step should definitely be reducing carbohydrate intake. The second step would be reducing protein intake to maintenance levels, and therapeutic fasts are very important. But the main thing, I would say is the omega 3 to 6 ratio, I believe that they should be an omega 3 to 6 index, just like with the glucose-ketone index, and they should work together, as a synergistic therapy.
Because you could even argue the ratio of omega 3 to 6 is even more important than the ketones. I would also say, the magnesium is very important with that too, those three things. Therapeutic ketosis, the omega 3 to 6 ratio and the magnesium I would say are very important for brain cancer patients.
[Damien Blenkinsopp]: Great, thank you, that’s some great takeaways for people at home. Andrew, I’ve got to say this has been really amazing interview – it’s amazing all of the different avenues you’ve run-down and all of these different aspects that you found to improve your situation. I know it’s going to be an inspiring story for the audience.
Thank you very much for being on the show.
[Andrew Scarborough]: No problem, we did cover a lot but we got there in the end.