Life extension – this is something I’ve wanted to spend time on for a while.
In this episode, I interview 5 thought leaders from the life extension movement. Consider this an introduction to the current status of life extension tools and technologies, as we look at most areas with a broad first-look.
You will learn where things are and what the risk profile of those life extension tools and technologies is today.
All interviews took place at RAADfest in San Diego. This is one of the larger life extension technology conferences today. It stands for Revolution Against Aging And Death and then fest for the festival.
I would encourage you to skip around this episode. It’s long, and there might be a specific topic that you’re interested in. So check out the notes below and pick the area that you’re most interested in first and start there. If you get through the whole thing it will give you an overview of where things are currently at.
“At the moment we’ve got this burgeoning of the rejuvenation technology industry with more and more investors realizing that this is the next big thing”
-Aubrey de Grey, PhD
“Our life is code and I think that we can modify that. First, we’ll look for human health and then we’ll look to enhance your life for where you want to live, who you want to be and what you want to achieve.”
-Liz Parrish, CEO of BioViva
“Basically what we’re trying to do is reproduce the young physiology that you had when you were younger [by replacing your old plasma with younger plasma]”
-Dr. Howard Chipman
“It’s not entirely crazy to think that some point soon, we can turn some of these senescent cells back into healthy cells.”
-Brian M. Delaney
“Not all biohackers would call themselves quantifiers. […] In the quantification side, well instead of taking 20 things, if there are two or three I can do that I get 90% of the benefit from, I’ll do that. That’s efficiency.”
-Bob Troia, “Quantified Bob”
The episode highlights, biomarkers, and links to the apps, devices and labs and everything else mentioned are below. Enjoy the show and let me know what you think in the comments!
What You’ll Learn
- Start of the first interview at RAADfest with Aubrey de Grey. Presentation of SENS Research Foundation. (9:32).
- The actual state of SENS Research Foundation. (12:22)
- Therapies to target the seven types of aging damage. Some of the diseases linked to them. (14:18)
- Companies associated with SENS and the variety of startups that have sprouted from it. (16:57)
- Aubrey’s particular views and interests in life extension. (28:10)
- Start of Liz Parrish’s section and the introduction of BioViva. (33:50)
- The new focus of BioViva, using a meta-analysis of public data to find promising drugs and genes (38:30)
- The scale and patients of BioViva’s potential gene therapy treatments. (41:30)
- The biomarkers Liz and her group work with, where they come from and how they are detected. (44:00)
- The process of receiving a specific gene therapy (1.0 vs 2.0 human) (46:00)
- Self-experimentation, data collection and associated biomarkers (48:41)
- What drove Liz Parrish to investigate riskier and more experimental medical areas. Her initial experiences in the area. (53:00)
- The process and the legal loopholes that were necessary for Liz to be treated with gene therapy (56:00)
- The current treatments and products BioViva offers. Future prospects for genomic counseling, new genes, and methylation testing. (59:48)
- Ending of the interview and Liz’s conclusion on the potential of gene therapy (1:00:50)
- Start of the interview with Howard Chipman, from Young Plasma (1:02:15)
- The basis and origin of the Young Plasma Project. (1:05:08)
- The positive and negative effects of using Young Plasma and the protocols associated. (1:07:21)
- The Ambrosia study and the biomarkers that are generally used in Young Plasma (1:08:30)
- The cost associated with participating in Young plasma and the mechanisms of the process. (1:11:34)
- Howard’s own experiences in the area and ending (1:13;40)
- Start of the interview with Brian M. Delaney. His experience with Young Plasma. (1:18:20)
- The introduction of Brian M. Delaney and his work in the LEF (Life Extension Foundation) (1:22:37)
- The repurposing of old drugs for new anti-aging purposes and new treatments and research. (1:24:20)
- Brian’s objectives in LEF and life extension (1:30:00)
- How Brian got involved in the area of life extension. (1:32:43)
- The current state of Brian’s research. (1:36:00)
- Brian’s health, tests, and biomarkers. His experiences with Calorie Restriction. (1:41.10)
- Further experiences of Brian with CR, insomnia and other physiological parameters. (1:51:10)
- Brian’s experience with Rapamycin, nicotinamide riboside. (2:02:01)
- Brian’s experience with Metformin and senolytics. (Dasatinib and Quercetin). (2:08:32)
- The effects of senescent cells in our body and the off-target effects of senolytics. Senomorphics. (2:13:59)
- The DNA methylation testing at Zymo Research Program. (2:19:39)
- End of the interview with Brian M. Delaney. (2:23:34)
- Start of the interview with Bob Troia (Quantified Bob). Presentation and opinion of RAADfest. (2:24:44)
- Bob’s activities, tracking during the last few years. Recent changes in the landscape of life extension. (2:28:39)
- Which consistent data in Bob now regularly collecting about himself. (2:38:12)
- Ketone testing and Bob’s experience with KetoneAid. (2:40:11)
- Recent advancements and curiosities in the area of life extension and supplementation. (02:46:57)
- End of the interview with Bob Troia. Invitation to contact him through social media and his web. (2:50:10)
- Damien’s conclusion and some questions to take home about the main themes of the podcast. (2:51:13)
Thank the interviewees on Twitter for the information they shared and let them know you enjoyed the show.
Thank them here: Raadfest (the conference), Aubrey de Grey, Liz Parrish, Brian M. Delaney and Bob Troia (Quantified Bob).
Interviewees in Order of Appearance
Aubrey de Grey, PhD
- Background: Aubrey is the de facto founder and head of the movement for life extension. He began working on in the 1990s. He is the head of SENS Research Foundation(SENS = Strategies for Engineered Negligible Senescence). Aubrey was previously interviewed in Quantified Body episode 14.
- Books: The Mitochondrial Free Radical Theory of Aging (1999) (Dr. de Grey’s first book on the subject of his Ph.D.).Ending Aging: The Rejuvenation Breakthroughs That Could Reverse Human Aging in Our Lifetime (2008). This last one provides a good primer/framework on the 7 areas of aging damage research.
- Research: Dr. de Grey’s research journal is Rejuvenation Research. For a comprehensive list of his research see Aubrey de Grey’s PubMed. A key paper is Strategies for engineered negligible senescence. It describes the strategies for engineered negligible senescence (SENS). It expands on the concept and provides the background to all his work.
Liz Parrish
- Background: Parrish is the CEO of BioViva, an advanced life extension center. It aims to develop new gene therapy based health testing and analysis techniques for the betterment of your health. They offer help navigating the details of genetics and family history. They can also assess how they impact your health and well-being.
- Self-experimentation: She was the first person to undergo gene therapy. In particular, one targeting life extension. This took place three years ago. She’s known as patient zero in some circles for this reason. Check Liz’s journey as a test subject of gene therapy here.
- Research: As CEO of BioViva, she recently presented the results from her telomere lab. Telomeres are DNA pieces we can look into to assess aging.
- Follow Parrish on Twitter.
Dr. Howard Chipman
- Background: Dr. Chipman is the medical director at Atlantis Clinic. He oversees the Young Plasma section. Their approach is to transfuse all the regenerative and healing factors present in young blood. This is done by transfusing the plasma (blood minus the cells) of young donors into an older patient. This was first tested in the 1920s in Russia. He is also involved in Aurora Aerospace. It is a space training company for jet fighters and zero-gravity flights.
- Research and experience: He specializes in emergency medicine. He has treated patients with life-threatening conditions. These include heart attack, drug overdose, shock, or massive bleeding. You can check Dr. Chipman’s Pubmed articles here.
- Find Dr. Chipman on Facebook.
Brian M. Delaney
- Background: Brian is an advisor for the Life Extension Foundation. LEF is a nonprofit organization. Their long-range goal is to extend the healthy human lifespan. This will be done by discovering scientific methods to control aging. They have been proficient in the supplements area. They have produced many well formulated and effective supplements. Before his involvement in the LEF, he was a philosopher and translator. He is based in Stockholm, Sweden. He is also a founding member of theCalorie Restriction Society.
- Books: The Longevity Diet is Mr. Delaney’s most popular book. In here he and Lisa Walford explain in practical terms the concept of calorie restriction. They consider CR “a life-extending eating strategy with profound and sustained beneficial effects”.
Bob Troia (Quantified Bob)
- Background: Bob appeared in episode 22 way back in the Quantified Body. He quantifies a lot of n=1 experiments and publishes them on his blog.
- You can find him on Twitter.
Tools & Tactics
Interventions
- Stem cell treatments to combat cell loss. Stem cells are undifferentiated cells capable of generating many different cell types. They substitute the ones lost through aging1.
- Mitochondrial mutation treatments to combat aging. Still in the early stages. Mitochondria are cellular organelles responsible for energy production. The accumulation of mutations throughout life can impair them. It can even stop their correct functioning. The reversal of these mutations might partially stop the aging process.
- Telomerase induction therapy. Telomeres, the protective ends of linear chromosomes, shorten throughout an individual’s lifetime. Telomere shortening is a hallmark of molecular aging. It is associated with the appearance of age-related diseases. Several scientific articles, including María Blasco‘s 2 have been recently published. They suggest that telomere growth can reduce the phenotypes of aging.
- Myostatin inhibition therapy. The inhibition of this protein can increase muscle mass and strength. These results apply to mice3 and possibly in humans. It is believed that it could be successfully employed in cases of muscular dystrophy.
- Intravenous fluid therapy. Intravenous fluid therapy. It is the introduction of a fluid (plasma, serum, antibiotics) in the vein of a patient. It is generally for employed for purely medical purposes. In the case of Young Plasma, it is the method used to introduce the plasma in the patient’s system.
Tech & Devices
- 10,000 Lux Lamp: Lamp that replicates strong sunlight. Damien has been using this in the morning to reset the circadian rhythm. this has the result of improving sleep quality. These lamps are designed for use by people with Seasonal Affective Disorder (SAD). They provide sunlight in dark months of the year.
Supplements & Drugs
Drugs (Typically More Potent/ Require Prescription)
- Senolytics: They are small molecules capable of inducing the death of senescent cells. They are still under research. Senescent cells are non-functional ones. Dasatinib is a compound generally used in cases of leukemia. As of late, experts think it can be repurposed as a Senolytic along with Quercetin. Brian mentions taking 5.0mg of Dasatinib and 50 of Quercetin per kg of body weight.
- Metformin: A drug used to improve blood sugar regulation in diabetes. Researchers are looking at its wider applications with cancer treatment. It can inhibit insulin secretion. Brian mentions taking up to 500mg.
- Statins: They are lipid-lowering medications. They can reduce illness and mortality in those who are at risk of cardiovascular disease.
Supplements
- Rapamycin: A compound that has been researched for its life extension properties. According to Brian, it is potentially senomorphic (capable of restoring senescent cells). It is believed to work by stopping certain responses to nutrients that can accelerate aging.
- Nicotinamide riboside: Brian mentions that it is useful for raising NAD+ levels. This happens in particular in the blood and in the cells. NAD+ is used in many redox reactions, including the ones needed to get energy. Brian mentions taking up to 500mg daily at some points of his fasting cycle.
- Nootropics: They are drugs, supplements, and other substances. They might improve cognitive function in healthy individuals. In particular, they may improve executive functions, memory, creativity, or motivation4.
- KetoneAid: It is a series of ketone esters (beta-hydroxybutyrate). They possess a great energetic performance. Generally used by elite athletes to achieve great bursts of power.
- Ketosports KetoForce: KetoForce contains the endogenous ketone body beta-hydroxybutyrate (BHB). It is in sodium and potassium salt form. The compound BHB can be used as an energy source by the brain when blood glucose is low. Ingesting KetoForce raises the levels of blood ketones for 2.5-3.0 hours after ingestion. (Note: A similar product from the same company is Ketosports KetoCaNa). Damien expresses his preference for KetoCaNA.
Tracking
Biomarkers
Inflammation Markers
- High Sensitivity C-Reactive Protein (hs-CRP): Elevated hs-CRP levels show inflammation. That is damaging to inner artery walls. If your level is below 1 mg/L then you do not have a cardiovascular disease risk. Liz mentions this as an example of a classical biomarker.
- Homocysteine: High levels can be predictive of increased risk of inflammation of blood vessels. Low levels are generally not indicative of anything in particular. Anything over 150 μg/dL is generally considered an elevated concentration.
Blood Sugar Regulation Markers
- Fasting Glucose Levels: A biomarker used to understand blood sugar regulation. Optimum levels are between 70 and 90 mg/dL. Higher ones show some level of blood sugar dysregulation. That lack of regulation increases the risk for diabetes II. Liz mentions this again as a classical biomarker.
Cholesterol Based
- Low-Density Lipoprotein (LDL): The traditional measure of ‘bad cholesterol’. That is the type that causes heart disease. Less than 100 mg/dL is considered an optimal level. Levels between 160-189 mg/dL increase the risk of cardiovascular disease. Research has shown that LDL alone is not the best predictor for cardiovascular risk. LDL particles with the smallest sizes are most damaging to the cardiovascular system. Still, as Liz says, people with high LDL might never have a heart attack.
- Testosterone: It is the primary male sex hormone and an anabolic steroid. Testosterone is used as a medication in several cases. Some of them are low testosterone levels in men and breast cancer in women. Normal levels are between 264 to 916 ng/dL from 19 to 39 years old males, and they decline after that.
Associated to neurodegenerative diseases
- Amyloids: Amyloids are proteins that can arrange into fibers and plaques in the brain. They give origin to diseases like Alzheimer’s. The presence of visible aggregations has been associated with the origin of the disease. Still, recent studies might show that it is not the plaques that are responsible. Individual free proteins might cause the disease. Several complex methods that use specific ligands are used to detect them5.
Associated to cancer
- Carcinoembryonic antigen (CEA): It is a set of proteins that are mainly present during the fetal stages of development. This is why their presence in normal blood is usually very low (about 20 ng/mL). Still, these levels increase in some types of cancer, which is why it is used as a tumor biomarker.
Lab Tests, Devices and Apps
- Magnetic Resonance Imaging (MRI): Mainly used to provide information on the inner workings of the body. Liz used MRI throughout her gene therapy to view any changes in muscle mass and white fat.
- Telomere length testing: Telomere shortening is associated with many health conditions. These lengths can be altered in response to social and environmental exposures. These two discoveries have underscored the need for methods to quantify telomere length. Terminal restriction fragmentation is one of the main methods used as of now for this purpose6.
- Methylation testing: Methylation is a series of modifications that your DNA can be subject to. They play an important role in many chronic diseases. Through tests you can more effectively understand the diseases you might develop. BioViva aims to include this test to their list. This will enhance its predictive capabilities.
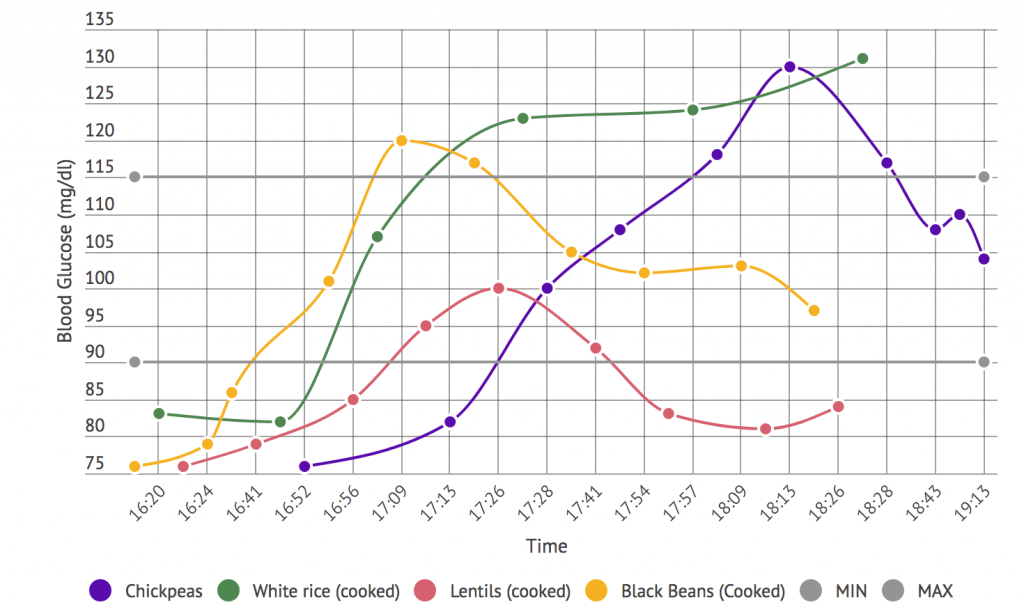
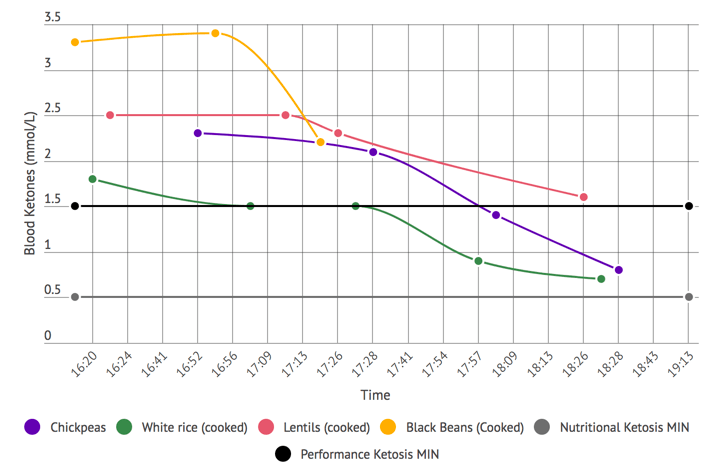
- Ketone testing: The different approaches to measuring ketones provide different perspectives on your ketone metabolism. These can be looked at as the ‘window of snapshot’ that they represent. Some methods have a snapshot of a longer duration. These provide more of an average reading. Others might provide a direct status of that exact moment. Moving from the more average-based value end of the scale to the more direct status end you have:
- Measuring ketones via the urine (via the ketone body acetoacetate). They have the longest snapshot with it representing your ketone values over the last 5 to 6 hours.
- Measuring via the breath (the ketone body acetone). It has a smaller snapshot window of the 2 hours leading up to the measurement.
- Measuring via the blood (via the ketone body beta-hydroxybutyrate). It provides you a snapshot of your ketone level at that exact moment.
The various devices available for glucose/ ketones testing and mentioned include:
- Urine Ketone Strips: Several parameters can interfere with the measurement values provided. They include both hydration status and becoming keto-adapted. They are the cheapest and starting with them is recommended.
- Ketonix Breath Meter: Currently the only breath acetone meter. If you are moderate to high on this meter you are in ketosis (i.e. typically over 0.5 mmol/L). This device is recommended in epilepsy cases.
Other People, Books & Resources
People
- William Faloon: The actual president of the Life Extension Foundation. Check his twitter here.
- Dean Ornish: An American physician and researcher. He is the president and founder of the nonprofit PMRI. That stands for Preventive Medicine Research Institute in Sausalito, California. You can check his website here.
- James Clement: The founder of Betterhumans, a transhumanist bio-medical research organization. They run open-label, non-randomized simple controlled trials
Organizations
- SENS Research Foundation: Foundation for the research of “Strategies for Engineered Negligible Senescence”. Founded by Dr. de Grey as an offshoot of The Methuselah Foundation. They work to develop, promote, and ensure widespread access to therapies. In particular, those that cure and prevent the diseases and disabilities of aging. This is done by repairing the damage that builds up in our bodies over time.
- Ichor therapeutics: It is a vertically integrated pre-clinical contract research organization. They focus on the study of aging and aging pathways. It was set up to work on macular degeneration, which is the number one cause of blindness in the elderly.
- Covalent Bioscience: It sprouted out of the work that SENS funded on amyloidosis. Amyloidosis involves waste products accumulating outside of the cell especially in the heart. They aim to develop and create affordable, better antibodies and vaccines. These will aim to solve a range of unmet medical needs.
- Unity Biotechnology: Flagship company in the area of removal of senescent cells. Their mission is to extend human healthspan, the period in one’s life unburdened by the disease of aging.
- Juvenescence: It is a drug development and artificial intelligence company. It focusses on aging and age-related diseases. It was created by Jim Mellon and his colleague Greg Bailey. Juvenescence AI combines advances in artificial intelligence with classical development expertise.
- Andreeseen Horowitz: It is a venture capital firm in Silicon Valley, California. It backs bold entrepreneurs building the future through technology.
- Y Combinator: It is an American seed accelerator, started in March 2005. They select and fund startups with great potential to allow them to grow as fast as possible.
- BioAge: A company started by Christian Foley. It focuses on using a unique computational platform that explores a universe of proprietary and public data. The main aim is to identify and target molecular factors that influence longevity. Their target is to slow and stop aging.
- Insilico Medicine: A company run by Alex Zhavoronkov. It specializes in the field of deep learning for drug discovery. It is also invested in personalized healthcare, and anti-aging interventions.
- Integrated health systems (IHS): A company focused on advancing the healthcare industry. They do this through the latest Gene Therapy techniques used in longevity research. By pulling from public sets of biomarkers they aim to select some to identify patients. These patients will then receive the gene therapy treatments.
- SpectraCell: A group of laboratories specializing in personalized disease prevention and management solutions. They were used by Liz Perrish for the MRI imaging and the telomere length testing.
Resource Links
Here are the links to each individual interview on our facebook page. On top of that, there are other interviews that weren’t included in the podcast:
- Aubrey de Grey
- Liz Parrish
- Howard Chipman
- Brian M. Delaney
- Quantified Bob
- David Wood: Chair of London futurists. Author of Sustainable Superabundance.
- “Reason”: Founding member of Repair Biotechnologies.
- David Bowman
- Mariana de Chellis: Member of Outreach. Check her Instagram here.
- María Entraigues-Abramson: Founder of Longevity bridge. She is also the Global Outreach Coordinator for the SENS Research Foundation.
Full Interview Transcript
You may know that that’s been a personal interest of mine for quite a while. This podcast is basically looking at topics of life extension, longevity, performance and general wellness and how we can quantify and ensure that we’re getting those types of results.
So this is something I’ve wanted to spend some time on for a while and you could look at this as an introduction to the current status of life extension tools and technologies and where things are and what you could do an experiment with today and what the risk profile of those tools and technologies could be today. Or actually the potential quantified benefits, if any.
So this is a test episode because basically it’s based on some live videos that I recorded with people attending RAADfest 2018 which was held in October in San Diego. RAADfest is one of the larger life extension technology conferences today. RAADfest stands for Revolution Against Aging And Death and then fest for festival.
So pretty much everyone who is active in this new industry, companies like Life Extension Foundation, the hosts and the leaders of this conference, Coalition for Radical Life Extension, investors, biotechnology startups in this new industry which is called Rejuvenation Biotechnology. That’s the name it’s starting to get for itself. All of these people were here at this conference so you’ll see there are a number of different profiles that I interviewed and that you can find in this interview.
So I think it’s a good episode to get an introduction into these topics to start understanding where life extension is and start getting an idea of where you may want to look into more and learn more about one of these topics. If you want to go check out the live videos, those are all on the Facebook page. So you can go to Facebook and just search the Quantified Body and you’ll find all of these interviews in the live videos there.
I would encourage you to skip around this episode. It’s long, as I said. So if there’s a specific topic that you’re interested in, you may want to check out thequantifiedbody.net blog and check out as always, we have the highlights, the times, who’s talking about what subject at what time in the episode so you may want to just jump to one hour or two hours in. Pick the area that you’re most interested in first. However, going through the whole thing will give you an overview of where things are at.
So with that, just let me give you some brief introduction into the topics and the people who are going to appear in this episode.
The first one is Aubrey de Grey from SENS Research Foundation. I interviewed him in episode 14 of The Quantified Body podcast. Really in this episode, he gives us an update on how life extension has moved from the fringe, basically something that was looked at as a fringe science, to becoming a new biotechnology industry where you know have a lot of funding coming in and a lot of startups becoming active.
As I said before, this is now starting to become labeled, rejuvenation biotechnology. I just went to another conference on this in London just a few weeks ago where there were a lot of prominent people and investors. So you can really see that this is growing into an industry all of itself more credible. So that was a good discussion on the progress of the tools and the funding and everything that’s going to bring it alive and make it happen in the longer term.
The next person I interviewed here was Liz Parrish from BioViva. Liz runs a biotechnology company focused on life extension and she was the first person to undergo gene therapy targeting life extension and this took place three years ago. She’s known as patient zero in some circles for this reason. She just presented the results from her telomere lab. Telomeres are something that people are looking at to measure how we age.
The idea is that telomeres get shorter as we age so you can have an idea of someone’s biological age based on measuring the length of your telomeres. So hers were actually shorter than average when she first tested before her gene therapy and now they are longer than average three years down the line using the same test from SpectraCell Labs to measure that. So with Liz, we talked about plans for her company to support the development of life extension therapies and of course her own experience with gene therapy to extend life.
The next person we have on the show is Reason from Repair Biotechnologies. So this is one of the new biotechnology companies that has emerged and been funded in this area already and they’re working on life extending therapies. He’s also the author of the blog Fight Aging which has been around for a really long time.
I’ve known about this blog for a very long time and he’s constantly been covering the science, the updates and how things are progressing; the ideas, tools and so on. So it was interesting to talk with him about his own self-experiments with senolytics, which you’ll learn about is probably the newer term tools that people will be using to aim to extend or rejuvenate themselves and also just an overview of where he’s focused and the science he has covered and some of the more interesting things.
The next person is the episode is Brian M. Delaney from Life Extension Foundation. So Life Extension Foundation, you may know of, is a company that has been very active in the supplements area and they tend to have better formulated supplements than the average company and they’ve always written pretty good articles with in depth references and citations. So Brian is sort of chief guinea pig for the life extension which is his new role he has taken on. He has been an advocate and someone who has practiced caloric restriction for a long time.
So we talked a little bit about that and then we talked about his new job with Life Extension Foundation and the things and the tools he has been testing which include senolytics and Rapamycin; two potentially newer term tools that can be used for longevity purposes to try and extend your life. Also go into depth in both of those and his own experiments on what he has been up to.
Next person on the show is Quantified Bob, Bob Troia. So Bob appeared in episode 22 way back in the Quantified Body. He does a lot of n=1 experiments and he quantifies those so obviously he’s a good fit for this podcast so you might want to go back and check that. Basically, we had a chat about what he found interesting at the RAADfest, which of the life extension topics he’s most interested in and also his other recent quantified experiments that he has done since we last spoke to him.
And finally, the last person in this episode is Howard Chipman from Young Plasma. Now Young Plasma is providing transfusions today of young blood so blood from young adults to people who are older in order for them to benefit from rejuvenating properties. This was first tested in the 1920s in Russia in fact.
Since then, there have been mice experiments and there has also been some allozymes as human studies which have shown benefits from basically just transfusing younger blood into people with older blood. So he talks about that service, he talks about the latest study , Ambrosia, and how he got involved with it and what patients are doing and who’s using this currently. So that’s obviously interesting therapy right there also.
As per usual, there are extensive show notes for this episode. They may be more useful than usual. There’s links to everything mentioned in the show including the studies and easy listed takeaways. There are summaries of the biomarkers, the tracking, the tools and the tactics we discussed in this longer episode.
So please reference those especially if you’re not sure about anything. I know some of the topics get a little bit deep in this episode because some of the topics like senescent cells are actually complex. So I think you might find some of the show notes useful to get up to speed there.
Also if you want to receive in future, updates on episodes and so on, go to thequantifiedbody.net forward slash newsletter and from then on and henceforth, you will get an email from me in your inbox whenever a new episode comes out with all of the details of that episode. So you won’t even have to go to the blog.
That’s it for me. I’m now going to leave you to delve into these episodes and get a broad introduction into the topic of life extension.
[MAIN INTERVIEW TRANSCRIPT]
(00:09:32) [Damien Blenkinsopp]: There we go. We’re live again. We’re at RAADfest again and we have Aubrey de Grey sitting next to us which is fantastic. If you’ve been watching the podcast, you’ll probably know that we spoke to Aubrey de Grey in episode 14 which was about three years ago I think. So we’re not going to go over all of that stuff. If you want to get up to speed on the basics and what he’s doing, check that out later and then you can come back to this. That’s probably the best way to go about it.
We want to talk about what’s going on now, what you’ve been achieving and then how it’s all going. So first of all, we didn’t talk a lot about the SENS Research Foundation; how it’s structured and basically what the mission is and how it’s structured to achieve that. So I thought that would be a good place to start.
[Aubrey de Grey]: Yes it is. If you [check 10:12], first of all, just generically, but also because that has been changing over the past couple of years. So we are based in California and we’re a charity. We’re a 501c3 as it’s called in the U.S. and that means that people can give us money with tax advantages. We also incidentally have an affiliate charity in the U.K. so that U.K. taxpayers, ID taxpayers from most of Europe can do the same.
But our goal is not only to get work done internally on the basis of money given to us, but also to be the engine room of the industry. Of course you might think well what is this industry? There has been this thing called the Anti-Aging Industry for quite some time, but it doesn’t have a very good repute. That’s no surprise because it’s fundamentally based on things that don’t work or hardly work. We are creating. We’re the new industry; the Rejuvenation Biotechnology industry [unclear 11:05].
[Damien Blenkinsopp]: You renamed it.
[Aubrey de Grey]: Things that do work. That’s right. Now that has really only happened over the past couple of years. There have been investors coming to us saying, “What can I do? How can I get involved in this? But I don’t like giving money away so please give me an investment opportunity.” Historically, we would not have been able to help them because the projects that we were working on were too early a stage for us to be able to make a case that really joined the dots all the way to eventual profitability.
That is no longer the case. We’re now up to about half a dozen projects that we gestated for, in some cases several years, and that we eventually were able to spin out and to start up companies and every one of those companies is doing pretty well in terms of bringing in money. In some cases, money that is the equivalent of multiple years of our entire annual budget.
The foundation is still very small. We only survive on something like five million dollars per year. Some of these companies are getting twenty or more and that’s fantastic because it means the science can get done faster. It’s also fantastic in the sense that we can focus on the projects that are lagging behind and still have not reached the point where they can be spun out and made interesting to investors.
(00:12:22) [Damien Blenkinsopp]: Yeah. So is that transformed over the last three years?
[Aubrey de Grey]: Really, yes. Until, I’m going to say four years ago, we had never done this. Not only we had never done it, but at the moment we’re in a position where we’ve spun out six companies I believe now, but actually we’re also working closely with at least a dozen or more other companies.
They’re not spin-outs, but they’re doing very closely aligned work and the people are very much looking to me and the foundation as source of introductions to investors for example. So for me personally, it’s extremely gratifying. I’m able to maintain this position of influence in the emerging industry that I historically had in the non-profit world.
[Damien Blenkinsopp]: So this is fantastic. So you listed several companies, the twelve companies that you spun out yesterday and also the SENS aligned. How many are there in total now that you consider within the right parameters?
[Aubrey de Grey]: Yeah. It’s a continuum. It depends how much [unclear 13:19] but at least a couple of dozen.
(00:13:22) [Damien Blenkinsopp]: Wow. Wow. We’ll get into some of the specifics of that. So one of the things I wanted to talk about is when you published your book. Was that 2008? The first year?
[Aubrey de Grey]: 2007.
[Damien Blenkinsopp]: 2007 and you published the seven types of damage of aging?
[Aubrey de Grey]: That’s right. I had been talking about that for at least five years before that.
[Damien Blenkinsopp]: Yeah. Last night, you said that basically that hasn’t changed. That model has withstood time.
[Aubrey de Grey]: It has withstood the test of time, that’s right. Always though was the risk that there could be some new type of damage that had not been discovered.
[Damien Blenkinsopp]: Yeah.
[Aubrey de Grey]: Of course there still might be, but every year that goes by when it’s not discovered is increasing circumstantial evidence that it’s never going to be.
[Damien Blenkinsopp]: Yeah.
[Aubrey de Grey]: Similarly with regards to therapies, it’s very important also to recognize that we have not had any bad news of the form of this or that approach that we thought we would be able to take to succeed in repairing this particular type of damage is not going to work for some reason.
[Damien Blenkinsopp]: It’s not dead end.
[Aubrey de Grey]: That has not happened either.
(00:14:18) [Damien Blenkinsopp]: Excellent. Excellent. Ok so if you got these seven areas, where are we making progress with this portfolio of companies now? Are there specific areas where we’re making progress now?
[Aubrey de Grey]: So that’s a much better finding. Really all of them, the progress is really encouraging; much faster than it used to be. So there is a possible big spectrum in terms of how far along they are. In fact, there has always been that spectrum.
So one of the areas is stem cell therapy to repair cell loss; cells dying and not being able to be magically replaced by cell division. That’s an area which was already sufficently established when we began a decade ago, but we have always deprioritized it with just an occasional little thing in the stem cell area. But other people with good money and from other sources are doing it so that’s [check 15:04] there. But pretty much all the other areas we have worked in, we have done quite a lot and yes they’ve all moved forward.
So the only one that is entirely within the foundation still is mitochondrial mutation. Even there, it’s probably not going to be all that long before we can [check 15:23]. Because after maybe ten years of working on it without anything really to show for it even before we were publication, we started making breakthroughs. We had our first real groundbreaking breakthrough publication two years ago now and we’ve made massive progress since then. We are universally recognized in the field as the world leaders in that area now and we believe that it’s going to be ready for private sector prime time fairly soon.
Now, that doesn’t necessarily mean that we can shut up shop and declare victory at the foundation. Because first of all, we are obviously doing other stuff in addition the research. We have this very vibrant education arm and also we do regular outreach. But also, even though some examples within these seven things are already out there in the private sector, it’s been out, nevertheless there are other examples that still need to be gestated for a bit longer before they can really be of any proposition.
(00:16:16) [Damien Blenkinsopp]: So some aspects of that damage hasn’t been spun out yet. So you said some of the mitochondrial mutations are looked at internally. When you’re saying internally, does that mean that you’re funding internal research or you’re funding external researches that you think are appropriate, but it’s internally funded?
[Aubrey de Grey]: In that case, it’s actually literally internal. We do the work in our own facility in Mountain View, California. We have a couple of other projects in Mountain View, but most of our work I think will be [check 16:44] about two-thirds is funded extramurally. In other words, we support professors in laboratories and institutes and universities.
(00:16:52) [Damien Blenkinsopp]: Wow. Ok, cool. Ok so if we look at the timeline, this is the kind of stuff people are going to be really interested in. If we look at the timeline of where these companies are and where you think they’re going to get to some commercial or even clinical trials or something that people could actually get involved in, could you paint a rough picture or maybe something we can expect?
[Aubrey de Grey]: Sure, absolutely. Absolutely. So let’s take Ichor. I would say out of all the actual spin-outs that we’ve had, that’s probably the poster child in the sense that it’s the one that has attracted the most funding so far and it has also grown in terms of the diversity of things it works on. Ichor was set up to work on macular degeneration which is the number one cause of blindness in the elderly. It’s an example of what we call LysoSENS. It’s caused by the accumulation of waste products inside the cell in a particular part of the cell called the lysosome.
We developed a method to fix that in house in our Mountain View facility. For several years, we couldn’t quite get there. We ran into the sand for a long time and we were a bit frustrated and one of our employees decided that he wanted to run with it. He felt he had a solution to this last problem. He was right it turns out [check 18:02]. He formed his company; fine with us.
We only took a very small nominal percentage of the company in return for the intellectual property. The technology went forward, they’ve got good money and there and then, they’ll be doing clinical trials next year or possibly even by the end of this year. That’s just one example.
Another company Covalent Bioscience which is in Texas. It’s a company formed out of the work that we funded on amyloidosis which involves waste products accumulating outside of the cell especially in the heart. It’s a very important phenomenon in terms of mortality and the [check 18:38]. That went well enough that the two main academics who were spearheading that work have now quit and gone full-time with the spin-out company. They are again hoping to be in clinical trials in the very foreseeable future so it’s happening.
[Damien Blenkinsopp]: Yeah. It’s starting to get to meet the road. Which do you think is going to be, I guess it’s the mitochondrial mutation which is going to be the last thing.
[Aubrey de Grey]: I don’t like to say. At this point, I would say the mitochondrial mutation strand is probably moving as fast as for example, the extracelular crosslinking strand; the [check 19:14] problem. The [unclear 19:15] problem is being spun out right now. It will be out within the next month. It just came together a little bit more quickly.
But I wouldn’t necessarily go on a rant in terms of how far along they are or how soon they’re going to be in the clinic. It’s all neck and neck. That’s how it should be. We have always been very careful to prioritize the ones that are at the most difficult, most challenging, most neglected so that they’ll catch up.
(00:19:43) [Damien Blenkinsopp]: So I was thinking about the seven types of damage. Liz Parrish, she has done one type.
[Aubrey de Grey]: Well two really.
[Damien Blenkinsopp]: All right, two types of [check 19:52] so that covers two areas of damage?
[Aubrey de Grey]: Yeah.
[Damien Blenkinsopp]: Ok. Basically you’re going to have people which are covering some of the damage, but not some of the other damage and it’s a bit difficult to understand what that may look like.
[Aubrey de Grey]: We have to give our finger on the past [check 20:07] very carefully because you’re right, but the utility of this taxonomy, the seven-point plan that we have must never be lost sight of. The utility comes down to the fact that for each strand, even though there may be many examples of a problem within the strand, for each strand there is a generic therapy. So if you have cell loss, it’s just stem cell therapy.
Now, different organs have different cell types and they need different stem cell therapies. So if you get one working, that’s not the end of the story, but it is kind of halfway to the end of the story because the stem cell therapy, even though they’re different, they have an awful lot in common. That means that once you’ve got a couple of them working then getting the next one working is going to take much less effort and much less time. There’s much fewer unknowns so we can push that forward.
It also means that it’s easier to make a case whether to scientists or to investors that this is something that they can make money out of in a timeframe that they’re comfortable with.
[Damien Blenkinsopp]: So in a sense once you’ve made progress in one of these areas, you’ve gone to clinical trials and you prove that even if it’s one-tenth of the actual end-output you need for that area, you’re validated, you’ve got credibility and that will make it a lot easier.
[Aubrey de Grey]: Let me also emphasize that you don’t necessarily even need to get as far even as clinical trials. So the strand of SENS that has been most in the news in the past couple of years is definitely senescent cells; removal of senescent cells. In that case, the company that’s really the flagship in this area, Unity Biotechnology, which is somewhat associated.
We could not describe them as a spin-out from us, but some of the founders have worked with us and have been funded by us. That company was able to attract its first [check 21:48] respectable enough like mid seven digit money on the basis of ridiculously preliminary data. Not just that it wasn’t clinical. It was only in mice, but also it was genetic models of mice that gave no particular reason to expect that one would actually be able to create drugs. It was even accelerated aging model which are always unreliable and they still were able to make a lot of money.
Since that time, their data has improved. They’re now worth nearly a billion dollars so this is a big deal. They’re not going to start clinical trials until later this year.
(00:22:18) [Damien Blenkinsopp]: Wow! This kind of leads on to some of the names you have in terms of the investing companies were quite big. You’ve got Juvenescence and you’ve got Andreeseen Horowitz, some huge names in the BC world and also Y Combinator. Has that made a difference? Why did these companies or these funders come in?
[Aubrey de Grey]: It’s beginning to. So some of the, well really all of the really early investors when the industry just was starting to begin three or four years ago, were private individuals using essentially, well starting with their own money. Juvenescence is an example. Jim Mellon and his colleague Greg Bailey, both very successfully invested in other areas and decided to get really into this. Other just private individuals decided to start their own thing.
It wasn’t so much a movement at the investor side of things at that point. But then after a year or two of that, things started to change. So Andreessen Horowitz, obviously as you said an extremely established name in BC, doesn’t do much Biotechnology. They still don’t. They decided to get into this area just because they’re with this one company, BioAge. Which again is not technically a spin-out from us, but we work very closely with them, that was doing bioinformatics. So Andreessen Horowitz is very heavily involved in informatics in general.
So it was just something that they felt that they could understand really and do well. They felt a bit comfortable with it, it looked promising and of course, they were right. The company’s doing extremely well. Then Y Combinator has got into this whole field more recently, just really in the past year. They have again, not had much influence on Biotechnology until recently. They decided to do that and furthermore, they’ve done it in a proper way.
They’ve done in a way that recognizes that Biotechnology just takes longer to get going than IT. So the typical deals that they would have had for IT companies would be more like three months to get to demo stage and then we’re only going to give you a few hundred thousand to create.
[Damien Blenkinsopp]: More effective products.
[Aubrey de Grey]: Yeah. Whereas when you get to Biotechnology, they recognize the difference in its order of events to mobilize the time and the money.
Yes, they are very much very clear that aging is a major preoccupation of theirs. They want to get into a startup landing in the biology of aging as quickly as possible. They’ve already got a few companies which again of course we’re talking to. They are [check 24:34]. They’re literally on the same street of us. They’re literally two blocks away.
[Damien Blenkinsopp]: Well that’s useful.
[Aubrey de Grey]: Yes.
(00:24:41) [Damien Blenkinsopp]: Ok so you just mentioned bioinformatics and BioAge. I don’t know if you’re allowed to talk about BioAge. I heard they’re more of a stealth mode.
[Aubrey de Grey]: They’re not really stealth, no. In fact, they share about what they know quite a bit, but what they have done as a result though actually of successful fundraising is they have been able to go broaden beyond the bioinformatics side. So Christian Foley who started BioAge is… she made a name at Stanford in bioinformatics. But the predictive ability that she was able to demonstrate with her original very small team of people was so good.
It mainly focused on metabolomics, but now spreading out to other onyxes. It was so good that the funding came in that was sufficient to be able to do their own lab work as well as to validate some of the drug candidates that they were identifying in silico. So now I’ve heard that a number of very good lab scientists are working at BioAge as well; again, friends of us.
It’s an extremely mission-oriented company. They’re very, very strong on making sure that they don’t get diverted by short-term investors into doing the wrong thing. That’s not true only of BioAge. It’s true across the board of the companies we work with.
Lessons have really been learnt here. A decade ago, you had a few cases of very well meaning, very smart gerontologists going out and forming companies and getting investment to actually take things forward. Even though it was earlier days in terms of science. A great example would be elixir, a pharmaceutical study by Cynthia Kenyon and Lenny Guarente. Complete waste of time, but it became a waste of time because they got the wrong investors. Because they got people on board who were much more interested in short-term [check 26:13] than they were in actual long-term success and the whole thing ended up being a total clusterfuck. That’s not happening these days.
[Damien Blenkinsopp]: Is it because you’re advising?
[Aubrey de Grey]: It’s a bunch of reasons. Firstly, it’s because the founders of these companies recognized that risk and they’re very careful of what money they take. But secondly it’s because the opportunity exists to take money from people who are not going to do that; people who really are high-risk high-rewards type investor types who are very comfortable with long-term strategies and yet who also have sufficiently deep pockets to be able to be the major investors for a long time.
(00:26:54) [Damien Blenkinsopp]: Yeah. Great. So you mentioned bioinformatics and I was wondering how important is that to the overall strategy? Because we especially saw [check 27:01] some of the data and the stuff they’re doing and I’m hearing more about that data. It’s obviously something that we talk about here for validation. Does that also have to be an area of investment to push this forward by being able to validate the discovery you were talking about with BioAge?
[Aubrey de Grey]: It certainly does and it’s not just validation either. Well a lot of it is, but the sheer ability to make predictions so that you don’t have too many things to validate is the key really. Another great example in our space is Insilico Medicine who also received a load of money and mostly from Juvenescence in that case. Again, run by a longtime and very ardent mission-oriented guy, Alex Zhavoronkov; great friend.
They are usually state of the art machine learning techniques to achieve really fantastic results in terms of prediction of not only new drugs, but also new activities of old drugs that could be repurposed and their aftermarket is shorter in that case. Yeah and they’ve been able to get very good investment.
I believe that bioinformatics will never do everything You’re always going to have to do a lot of bench work and everybody knows it, but it definitely has its place.
(00:28:10) [Damien Blenkinsopp]: All right, great. So I’d like to pass a little bit on to you actually because we chatted last time just about what you do. Do you do any tracking for yourself? Are you interested in any of these life extension? One of the things I’ve heard about quite a bit here is senolytics because some people see this as something short-term they can do to enhance their health spans and they can get to these technologies. What’s your view to this for yourself? Are you doing anything or are you interested? Do you think it’s not really worth it because you’re just waiting for the big stuff?
[Aubrey de Grey]: Everybody’s different in this. I always tell people, “Don’t do as I do; do as I say.” The reason I say that is twofold. First of all, I’m just well-built. I’m a really lucky guy. Well first of all, I’m lucky in that because of my providence in the field, I’m able to get for free the kind of really top of the range analysis of my metabolic state that would normally cost ten thousand dollars and I’ve done that maybe five times over the past fifteen years.
(00:29:04) [Damien Blenkinsopp]: What kind of analysis?
[Aubrey de Grey]: They measure 150 different things in your blood and all manner of physiological and cognitive tests; you name it, they do it. I always come out insanely younger than I actually am like fifteen years younger. What that means in terms of what I should do is I have to be very conservative. Respecting how little we really understand about metabolism. It’s a case of if it isn’t broken, don’t fix it.
So the fact that I actually eat and drink what I like and I don’t even do much exercise, nothing happens. I’m doing fine and so I might as well, but that doesn’t mean that I’m going to do fine forever. I always have to pay close attention to any early signs of something going downhill.
The other way in which I recommend people not do what I do is because of my position and my advocacy role, I’m constantly on the road. I definitely don’t get nearly enough sleep and that’s definitely bad for me. But I figured it’s probably [check 29:57]. I’m hastening the defeat of aging, but I’m [check 30:00] in my life.
[Damien Blenkinsopp]: Absolutely. Yes it’s really interesting because I’ve spoken to a variety of people here and they have got very different strategies. One person I spoke to, he’s basically stacking everything that you’ve seen here. Some of his markers, he actually isn’t in such great shape so the higher risk is worth it to him. But if you’re starting from a great place then as you said, until they’re proven, it’s not worth taking these things.
[Aubrey de Grey]: Precisely. Senolytics, for an example, the [check 30:27] is definitely one of the things in my seven point list and so I’ll definitely be willing to do that at some point. But at the moment, it makes sense for me to wait and see and let these therapies become more effective and more, you know, more tested. That’s happening so fast now that in one or two years down the road would make more sense to me.
(00:30:50) [Damien Blenkinsopp]: Yeah. It’s a very strategic unit. It really fits with what you’ve done with SENS Research Foundation. So this is the last thing. Where can people, I mean two things. Have you got an ask for the audience? Anything that you’d like to tell them?
[Aubrey de Grey]: Sure, totally! At the moment, as I said we’ve got this burgeoning of the rejuvenation technology industry with more and more investors realizing that this is the next big thing and it’s starting to come in too. But there is still this residue of projects that absolutely vitally need to be taking fold as well and yet are not yet quite at the point of investability even from the visionary end of the spectrum of investors. That’s why the foundation still exists.
Now the unfortunate part is that your average investor is not totally keen on giving money away. They got wealthy by not giving money away indiscriminately. Therefore if anything, the burgeoning of the industry side actually makes that much harder for us to bring money in philanthropically.
As such, we are still way short of what we need in order to go as fast as the difficulty of the science allows. I think we could still at least double the rate at which we make progress on the hardest and therefore the most essential aspects of this work. Absolutely I haven’t asked. I say anything you can do to help. We have a nice friendly donate button on our website, sens.org and if you want to give us more than that then you know where and how to contact us.
Other than that, if you’re not wealthy, you can still give us ten dollars, a hundred dollars a month; these add up. But also advocacy; very, very important. People who are not billionaires and not scientists may feel that they can’t do anything, but that’s not true at all because the quality of debates, the quality of understanding and discussion of this area is still being unbelievably strongly held back by the desperate need for most people not to get their hopes up about this.
This is what drives what I’ve called [check 32:47]. They hear rationalizations that allow people to trick themselves into thinking that aging is some kind of blessing in disguise. I get so frustrated that people just refuse to open their eyes because it’s holding us back. That lack of enthusiasm is making people not support this work financially. When I say people here, I don’t mean just individuals, I also mean companies and governments.
So shunting the course of debates just as you’re doing right now by having me on camera, this is what needs to be done.
[Damien Blenkinsopp]: Perhaps more of these conferences. More people attending the conference, getting more involved, more engaged.
[Aubrey de Grey]: Totally. RAADfest is growing. Yeah it’s a fantastic event. We also have our own event in Berlin every year, every March. The emphasis is a bit different. It’s more exclusively science at that conference, but the crowd is the same. The kind of connections you have, it’s across the whole spectrum from the hardcore scientists who are getting the work done at the lab through to all the advocates, the investors.
[Damien Blenkinsopp]: Aubrey, thank you so much for your time.
[Aubrey de Grey]: My pleasure.
[Damien Blenkinsopp]: It’s great to have you again. Yeah.
[Aubrey de Grey]: Thank you.
[Damien Blenkinsopp]: Can you go first? We were just talking about how we we’re going to talk and it just failed.
[Britton Schneider]: I’m Britton Schneider. I work with Liz at BioViva.
[Liz Parrish]: My name’s Liz Parrish and I’m the CEO of BioViva.
(00:34:15) [Damien Blenkinsopp]: You know me or you should do by now so I’m not going to introduce myself. This is going to be a great little chat based on some of the stuff I learnt yesterday from your presentation. Just talk about what BioViva is doing and also what you personally have done yourself which is one of the highlights. So first of all, just for the audience because many of them probably don’t know who you are and what you do. What do you do? Who are you?
[Liz Parrish]: I’m the CEO of BioViva. I’m considered the woman who wants to genetically engineer you. I want to create humans that are healthy and don’t die of the diseases of aging and therefore bring treatments back to children who are dying of critical diseases now that will cure them of their diseases.
[Damien Blenkinsopp]: That’s a really good introduction.
[Liz Parrish]: I’ve been doing it for a few years.
(35:00) [Damien Blenkinsopp]: So Aubrey de Grey just called you patient zero so you apparently have several names. Are there any others?
[Liz Parrish]: Well depending on who you talk to.
[Damien Blenkinsopp]: Good ones! Well if you get any bad ones. Any bad ones?
[Liz Parrish]: I don’t know of any bad ones actually. I don’t think that I get too much right now.
[Damien Blenkinsopp]: That’s good. Does Brit call you something? Does she have a pet name for you?
[Liz Parrish]: She calls me “you’re late.”
[Damien Blenkinsopp]: Ok.
[Liz Parrish]: That’s how I know myself.
(00:35:21) [Damien Blenkinsopp]: That’s the main thing there. Ok so what does BioViva do and what is its mission?
[Liz Parrish]: BioViva is a bioinformatics platform now. We’ve changed our gears. For two years, we tried to be a program that actually treated patients directly with gene therapy. We’re looking at regenerative medicine gene therapies; gene therapies that reverse the biological clock, gene therapies that create upregulation of regeneration in the body, gene therapies that increase muscle mass for the aging population and therefore creating cheaper cures for kids with muscular dystrophy.
So every one of the therapies that we talk about today, there’s an aspect that can be used in childhood disease. But we wanted to do that. We wanted to treat patients correctly, but we found out we couldn’t do that. There was not a regulatory framework for us to be a U.S. company and do that, but the most important part of treating patients is the data; what happened when a patient was treated.
So we actually became in partnership with an exclusive partnership with a company that’s offshore of the U.S. It can broker deals between patients and doctors to do gene therapy and we get access to all the pre and post data. We find out exactly what’s been done to the patient and then we look at the biomarker panel that we’re developing with our bioinformatics program and we see where gene therapies work and where they don’t work.
In research and development, we are actually starting to design our first viral vector that will get multiple genes in at one time.
[Damien Blenkinsopp]: So you are doing R and D still?
[Liz Parrish]: Yeah, we are.
[Damien Blenkinsopp]: Then you license that out, but you just don’t clinically deliver it?
[Liz Parrish]: No. The thing is you never want to fall in love with your hypothesis. So we don’t want to be a telomerase inducing gene therapy. We don’t want to be just a [check 37:06] inducing gene therapy, PCG-1 alpha, FGF21, Folistat. If you fall in love with your hypothesis, you’re going to try to prove that it works.
We’re a testing platform to see what works. We’re going to bring other companies through that have therapeutics that we will actually give them their first human data. So why would we do this? Why would we do medical tourism? It’s a multi-pronged approach.
Number one, you give patients access to therapies they couldn’t get otherwise. Often, these patients are in dire need of something and the regulatory system and their doctors would just let them die rather than treat them, rather than take the risk because we’re very risk-averse. So number one, you’re helping patients.
Number two, you’re helping biotechnology companies get the first data on whether their drugs work in patients and where they work and where they don’t work.
Number three, de-risking investment in biotechnology. Right now, biotechnology has a 94% failure rate through phase studies. Investors don’t want to invest, but if you plop down the data on ten, twenty, a hundred patients and what happened, we’ll know what drugs will work before we start to run them.
Do we think that drugs should go through a regulatory service? Absolutely. They should go through a regulatory service so they can be sold widely to a wider audience and help more people, but people need access now. The human model is the best model organism to work in to find out if drugs work for humans.
(00:38:30) [Damien Blenkinsopp]: So you completely pivoted the company. So before you were actually developing them and now you’re, just to get it straight, you’re not doing any R and D and development at all? Or you’re doing a bit, but mostly you’re going to be sourcing the R and D from other companies?
[Liz Parrish]: Instead of actually trying to run one gene to find out how well it works, we use the meta-analysis so it’s called bench to bedside. Where we are doing the development and research and development is the driver, the vehicle; what gets the genes into the cell. So we’ll let other gene companies and research institutions run all that expensive pre-data, but then we want to see what happens in patients when we look like we do have a promising drug.
[Damien Blenkinsopp]: So you’re going to select the most promising ones?
[Liz Parrish]: Yeah, that’s right. So the reason we would look at telomerase induction is it actually has decades of research done on it. Nobel prizes have been given out and fantastic, very inclusive research papers have come out. Maria Blasco just put out an exhaustive scientific paper about how telomerase induction does not cause cancer, it may actually protect against cancer. These are the things that we need to see, but if we don’t apply them to humans, they have zero value.
(00:39:42) [Damien Blenkinsopp]: So basically what you’re doing is you’re saying the regulatory environment is not going to let us do any of this and it’s very expensive to do the clinical trials. So we’re going to let less risk-averse people or maybe they’re in a situation where they’re at high-risk of dying or they have a very damaging condition already and so it’s in their interest to reduce risk. So they can do it for medical tourism then you can get the data and then fast forward and validation.
[Liz Parrish]: Fast forward those drugs. Actually, I think that our platform in the next two years, we’d like to prove ourselves and then we’d like to have the regulatory service look at our platform. If we actually ran drugs like we’re designing to run drugs, this is actually what we want. Don’t hide any of the data, show the data; where does it work, where does it not work.
That way we have a clear picture of what’s going to happen. We already take drugs that aren’t necessarily safe, but we’re none the wiser. We get a pamphlet, you get a bottle of statins, you get a pamphlet, but if you look at the Cochrane Report, a statin will save one in 164 patients from getting a stroke, but one in ten will get Type 2 Diabetes and one in 50 will get dementia from taking the drug.
We don’t understand our risks to begin with, but we’re looking at gene and cell therapies, we’re looking at just upregulating a beneficial protein that has decades worth of data on it in the human body to push regeneration. Not only may these patients actually recover from their disease if we’re lucky, they will be spearheading the technology for the future.
Our risk aversion just has developed so many myths around living as if we’re not actually going to die, but how is anyone actually going to solve the problem. Taking a gene therapy is the type of people who want to buy an experience, but they are also health investors; they’re investing in their future.
(00:41:30) [Damien Blenkinsopp]: You probably are talking to a lot of people who are interested in taking gene therapies, what type of people is this? Just to get some on the ground information. i’m sure these kind of people contact you. What kind of population are interested in this?
[Liz Parrish]: We get thousands of people who contact us and are interested in taking a gene therapy and they really span the gamma and some of them were excruciatingly heartbreaking earlier on because we didn’t have ways to treat patients. We had people come through with sick kids who have probably died since then because there was no option. People with muscle disorders, heart disorders and various really sick people. But also we get some pioneers. Some people that hands down would take any therapy to be part of the experience of spearheading technology for the human race.
[Damien Blenkinsopp]: Like some healthy people?
[Liz Parrish]: Some healthy people.
[Damien Blenkinsopp]: Like you?
[Liz Parrish]: Yeah, some not so healthy. Well if you look at biological aging, by the time I was 40, I’m not very healthy. These therapies will be used in sick people. We’ll see if we can regenerate a kidney, we’ll see if we can regenerate a liver, we’ll see if we can create some more beneficial cognitive effect in patients with Alzheimer’s. But then we’ll work them back to people in less disease state and soon, we’ll be using them as immunizations. How soon that happens is how fast we start working towards that data.
(00:42:52) [Damien Blenkinsopp]: So what is the timeline for this model you’ve put in place? Is it just started? Is it 2019 you’re going to have some clinics in specific countries in the world that’s run by this organization called IHC?
[Liz Parrish]: IHS?
[Damien Blenkinsopp]: IHS.
[Liz Parrish]: Yeah, Integrated Health Systems. Yeah so we’re starting now and already patients are signing up to talk to doctors. They are very interested in therapeutics so we’re hoping to start generating our data in 2019, but how clean that data is and what that data means is going to take us a little bit of time to generate. So we’re looking at a huge biomarker set. We’re looking at a multi-comeback…
[Damien Blenkinsopp]: There are four monstrous slides. I think I’m a data geek. It was ridiculous.
[Liz Parrish]: Yeah. So we’re going to pull from publicly available data sets, but we’re going to be analyzing, the first company in the world that analyzes what happens when you do regenerative gene therapies in humans.
(00:43:44) [Damien Blenkinsopp]: So you’re going to ask the clinics to collect this data? Because it was a very extensive amount. So do you need equipment like special MRIs?
[Liz Parrish]: Well we actually work with the doctor. So the doctors who are exclusive to IHS are actually exclusive to giving all of the data to BioViva.
[Damien Blenkinsopp]: That’s the new agreement?
[Liz Parrish]: Right, and there is protocol. So to every gene therapy, there’s a protocol, there’s a list of markers that have to be taken before a patient can be treated.
[Damien Blenkinsopp]: Ok.
[Liz Parrish]: It is pretty broad.
[Damien Blenkinsopp]: It wasn’t all of those though, was it?
[Liz Parrish]: Remember a lot of it is done in blood work. So a lot of those biomarkers come from blood work, DNA testing, methylation testing. Other markers come from imaging. So imaging is really important when you’re talking about brain health, when you’re talking about muscle health. When we’re talking about whole body health, we want to visualize what’s happening.
(00:44:38) [Damien Blenkinsopp]: Are you going to basically standardize the definition of the type of data and also how to record it?
[Liz Parrish]: Yeah, absolutely.
[Damien Blenkinsopp]: But who’s going to actually collect the data? Are you going to collect the blood samples and send it to a U.S. lab or a centralized lab? Or are there going to be labs all over the place or just the local ones?
[Liz Parrish]: So that depends on what labs the doctors work with, but they’re all the big companies. We work with generally the standardized labs.
[Damien Blenkinsopp]: Like [check 45:01]?
[Liz Parrish]: Yeah. Exactly. But we also work with some smaller companies that have some protein discovery methods, proteostasis, demethylations.
[Damien Blenkinsopp]: This specific test is more advanced.
[Liz Parrish]: Yeah so we’re not only looking at the old biomarkers that we used to look at c-reactive proteins and a blood glucose level, but we’re looking at these markers that will be really important in five years that really will be more specific than the other biomarkers in the coming years. That’s how we’ll find the real true biomarkers of aging that can give us a close date to the biological age of what your due date might be on your body and how we could actually change that.
But by doing regenerative therapies, we might be able to reverse engineer some biomarkers of aging as well.
(00:45:50) [Damien Blenkinsopp]: What does that mean?
[Liz Parrish]: It will give us a new view, a new insight of reversing pathology in the body and regenerating certain [check 45:58]. So for instance, even when you’re young, you’re actually generating damage. Your cells are degenerating in a slow form way. This isn’t just something that happens as you get older. Your body is developing so we have the illusion that we’re not accumulating damage, but in fact we’re accumulating damage over our entire lifespan.
We’ll be looking at bodies hopefully with regenerative medicine in these gene therapies that actually start to restore damage. That’s a reverse process of damage. Therefore we’ll get the insights of what that actually means with biological age. First, we’ll start pinpointing it back to a healthy body. A healthy what I call 1.0 body with a 2.0 body may have different biomarkers that give us insight to how to adjust to what is happening with aging in the body right now in the 1.0 body.
[Damien Blenkinsopp]: I’m not 100% following with this.
[Liz Parrish]: Sorry.
[Damien Blenkinsopp]: Sorry guys.
[Liz Parrish]: It’s probably me.
[Damien Blenkinsopp]: So 1.0 is someone.
[Liz Parrish]: 1.0 is a human who has not been given the gene therapy.
[Damien Blenkinsopp]: Ok. All right. So you’re saying once you get a gene therapy, you may not be normal? You might be something different, but it’s also healthy?
[Britton Schneider]: Ideally, yes.
[Damien Blenkinsopp]: Or it might be healthier?
[Liz Parrish]: You’ll be regenerating, well that’s what we’re hoping, is to put the body into a homeostasis; stronger, smarter, faster, healthier.
[Damien Blenkinsopp]: So that’s the 2.0?
[Liz Parrish]: Yeah. That’s any person who has gone through a regenerative gene therapy who has an upregulation of a protein that is designed to actually reverse damage in the body.
[Damien Blenkinsopp]: Ok so I’m following you now I think.
[Liz Parrish]: I nerded out.
[Damien Blenkinsopp]: The same way we’re upregulated with many detoxifications.
[Liz Parrish]: I went too far.
[Damien Blenkinsopp]: You talk fast. Not as fast as Aubrey, but he’s hard to keep up with. So for instance, we have many detoxification processes and enzymes in our body, you could upregulate some of those and then you could drink alcohol all day and not worry about it for instance, like Aubrey does.
[Liz Parrish]: Yeah, that’s true. That’s one use of our time.
[Damien Blenkinsopp]: Well I’m not saying it’s the best, but basically that’s what you are saying. We would have these abilities.
[Liz Parrish]: Yes of course. I’m all for people enjoying their life and living the life that they want to live.
[Damien Blenkinsopp]: We’ll go to the gym less and be stronger.
[Liz Parrish]: Yeah exactly. Well that was one of the things with my therapy. I worked out five days a week, I ran about 25 miles a week and after my therapy, I got on plane after plane, I had jet lag, I wasn’t working out. When we did my second MRIs, I was really worried because I had not been exercising, but the muscle mass was bigger, the white fat was down and my insulin sensitivity was up.
[Damien Blenkinsopp]: Ok Liz.
[Liz Parrish]: So that’s fantastic!
(00:48:41) [Damien Blenkinsopp]: I did want to talk about this of course I did. So on this podcast, on this show, we’re into self-experimentation so you’re a good fit and tracking data on it so that’s one of the key things. But I wanted to make sure we covered all the business and what you’re up to there because we’re also excited about the data. Because my belief and probably most of the people following the show which includes BCs, entrepreneurs, software experimenters and biohackers, is that data is one of the keys to everything because it will stop us running around in circles.
[Liz Parrish]: Yes, exactly and boy did we learn a lot about data. When we started this company, I found an investor. He said I’ll invest in you taking this therapy to embark on this and show that we can reverse biological aging. We have really big plans, but we didn’t really have a list of things that we really needed to do. So all I did was a lot of blood work, I did MRI imaging then I did telomere length. But today what we know is there’s so much more that we could do.
[Damien Blenkinsopp]: So you wish you knew probably more?
[Liz Parrish]: Of course, but that’s how you get there.
(00:49:38) [Damien Blenkinsopp]: What exact baselines did you take?
[Liz Parrish]: That’s when you saw my biomarker list, it’s extensive; it’s exhaustive.
[Damien Blenkinsopp]: Well because we don’t know which ones it’s going to affect.
[Liz Parrish]: No, we really don’t and we actually still don’t know what biomarkers [check 49:48] that we look at now. We’ve hunted LDL cholesterol like a witch hunt and yet people with high LDLs sometimes never have heart attacks.
[Damien Blenkinsopp]: I have high LDL, but I’m not worried about it because my particle count is low.
[Liz Parrish]: There is the group in Italy that have a gene. They never develop atherosclerotic plaques, but amazingly they have really high LDLs and then people with high HDLs and low LDLs die of heart attacks.
[Damien Blenkinsopp]: So it’s a perfect example.
[Liz Parrish]: So we have a long ways to go.
[Damien Blenkinsopp]: Because this biomarker is used everywhere and we don’t even know what it is.
[Liz Parrish]: Everywhere. Yeah.
[Damien Blenkinsopp]: It’s called bad cholesterol, but we really don’t know what it is.
[Liz Parrish]: So we need more data. We need to look at phenotype, we need to look at anatomical, physiological data. We have a long, long ways to go. So even before BioViva came along and started throwing regenerative gene therapies into people, we had a problem with biomarkers and we’re just pointing out that problem.
(00:50:40) [Damien Blenkinsopp]: Ok. So you’re going to collect a lot of data, but how are you going to get the value right because there are a lot of biomarkers. Are you going to put AI on it or what are your plans for this leverage?
[Liz Parrish]: Yeah, right now we’re using machine learning algorithms so our computer scientists and the PhDs that are working on that are trying to collect all of the best data and they’ll do a little bit of light machine learning as the data goes in. The most important thing is that the data is clean because garbage in, garbage out, we’re screwed. AI can’t solve a problem if we have no data.
AI is really fantastic for old drugs because we have a lot of data on how those work and it’s helping us understanding protein to protein interaction because we have some data on that. But regenerative gene therapies, we need human data then we can plug that in then we can start to get some meaning.
The microbiome, very interesting; changes as you age. If we are actually able to regenerate parts of the body, will the microbiome change? But we still don’t know most of the microbiome and we have issues.
[Damien Blenkinsopp]: Well I can tell you that I’ve done 40 different microbiome tests and I’ve never gotten actual information because you have to combine it Liz to get the real picture and assay islands.
[Liz Parrish]: Well it changes with what you eat.
[Damien Blenkinsopp]: It’s up and down all the time.
[Liz Parrish]: Yeah.
[Britton Schnieder]: We still have to identify what’s good and what’s bad. It’s still so much we don’t know.
[Damien Blenkinsopp]: Yeah, but we don’t know. There is a lot of “don’t know” basically so I think bioinformatics, it’s interesting. I’m always like, “Wow!” That’s what bioinformatics, and I’ve been thinking for a long time, we need to focus more on that. Because the more I get into, I’ve got into data just from this show and really it’s not accurate. A lot of this stuff is inaccurate. The more I’ve tested, the more I’ve spent on it, I’m like is this useful?
[Britton Schneider]: It’s the results.
[Liz Parrish]: Actually the arguments within the field. So in 2015, I took the two gene therapies that we’ll talk about. I did the telomerase induction and I did the myostatin inhibitor. Immediately people flew up and they were like, “Telomerase induction!” or they were like no, you should have tried this other thing. Well we have to get out and try these things. Without the data, we can’t say something doesn’t work.
(00:52:44) [Damien Blenkinsopp]: Could you talk about, I’m interested why did you take that decision to do it? Was it because you were frustrated the company wasn’t making progress?
[Liz Parrish]: No, no. The company actually was just starting. So in 2013, my son was diagnosed with Type 1 Diabetes. I was thrown into children’s hospital. I had been volunteering my time for two years working with stem cells and that advocacy and trying to figure out why the funding for stem cell had dried up and people weren’t interested when it seemed to show such promise.
So I had this regenerative medicine education that I was going through, I’m thrown into this hospital situation and I started asking them can you do something with stem cells. Could you biobank some of his pancreas so we can use it later and they looked at me like, “Lady! That’s experimental medicine.” They said kids are dying here. Your son has a treatable disease and I looked around and I saw that kids were dying and it was so unacceptable to me.
[Damien Blenkinsopp]: But your point is if they’re dying, let’s do something riskier.
[Liz Parrish]: Let’s do everything. Let’s do everything. So I left the hospital and I never really went home. I started getting on every board that I could get on as far as information on the internet, looking up what was going on and I found a SENS conference happening in England. That was 2013 and I got on a plane and I went over there and I said, “Ok what is what you’re doing, how does that help kids?” Because I was looking for treatments for kids.
I got there, they said, “Look, we’ve got all this great technology, we just need funding.” So if you look, I went home and I created a funding company. It was called BioTrove Investments. I started BioTrove podcast thinking that people just needed education. I’d get a call on the phone, I’d get to go fly around with fancy people who have a lot of money asking me a lot of questions about the technology. They said, “If you prove it works, I’ll put money into it.”
[Damien Blenkinsopp]: The investors?
[Liz Parrish]: So I said well ok two of my favorite things were telomerase induction and myostatin inhibitors because myostatin inhibitors were already working in humans. So I thought they will like this. So I found an investor and I said, “Let’s start this company and if you want to, I would take these gene therapies.” It will be my contribution to the world, it will be my contribution to my children and it will be my contribution to a world that I hadn’t really given much back to. He said, “Let’s do it. I think this will work.”
Of course we hoped to cure aging in one therapy, but we didn’t, but we got some really interesting data. We found out ok now we have to build the platform to make this a reality. Test every gene therapy that we can and see what combination is needed to actually achieve what we originally started.
[Damien Blenkinsopp]: So they gave you your start?
[Liz Parrish]: Yeah.
[Damien Blenkinsopp]: So how long ago was this?
[Liz Parrish]: That was in 2015.
[Damien Blenkinsopp]: So we’re three years on.
[Liz Parrish]: Yeah.
[Britton Schneider]: September makes three years.
[Liz Parrish]: Yeah, I took the therapy in September.
[Damien Blenkinsopp]: So it’s exactly three years.
[Britton Schneider]: Exactly three years ago.
[Liz Parrish]: Yeah, but the company was started January eighth 2015. The investor came in right away and then it took a long time to get that gene therapy. Then the gene therapy was delayed twice. So here I was ready and anticipating ok we’ll do it. We had considered treating a patient with it, but we couldn’t find any legal way to do that.
(00:55:57) [Damien Blenkinsopp]: How were you allowed to do it? I don’t really understand the regulatory.
[Liz Parrish]: There are some loopholes in regulations where if you are educated, you understand the product of your company, you can participate in the product of your company
[Damien Blenkinsopp]: It’s your personal company, that’s the view.
[Liz Parrish]: It’s not an actual law, but it’s a bit of a loophole and so the FDA never sent us…
[Damien Blenkinsopp]: But you have to be the owner of the company. Is that the thing?
[Liz Parrish]: Yeah and actually people have looked at ways to use that in order to sell shares in their company for people who want to participate in what their company is doing. So yeah spoiler, some people do that. It’s called making an educated decision. I’m a major shareholder in a company, it’s developing technology that will treat patients.
[Damien Blenkinsopp]: You needed that credibility to move forward.
[Liz Parrish]: I don’t know if it offered us credibility, but it sure ignited the industry. We were the first company to treat a patient for, or a person, in this case myself, for biological aging.
(00:56:57) [Damien Blenkinsopp]: Ok. So what baseline labs did you take?
[Liz Parrish]: We did all of the standardized blood tests that you would get at your doctor when you’re doing one of your uber health exams. We did MRI imaging, we did the telomere length testing.
(00:57:11) [Damien Blenkinsopp]: Which company was that?
[Liz Parrish]: We used SpectraCell. We actually used both SpectraCell and Life Length, but the Life Length one that we sent, they said they got it on the wrong day so they couldn’t analyze it.
[Damien Blenkinsopp]: You know what? One of my friends has the same problem. He stopped using them.
[Liz Parrish]: Yeah I was really like, “You are kidding me.” Actually, they were our company of choice. So at the last minute, we had to do a SpectraCell because they would take a 24 hour delivery at that point and we had to get it in within 24 hours because I was about to embark on the test.
[Damien Blenkinsopp]: That’s a shame you didn’t know.
[Liz Parrish]: Well what is great is one year after I took another SpectraCell and I went ahead and did Life Length again because they sent me a free kit because the first one got messed up. Guess what? They had the same value.
[Damien Blenkinsopp]: Exactly?
[Liz Parrish]: They pegged me at about 45 years old.
[Damien Blenkinsopp]: So the same as the SpectraCell?
[Liz Parrish]: Yes.
[Damien Blenkinsopp]: So the two labs coincided; that’s good.
[Liz Parrish]: They totally coincided. So the third one that we did this year, we used SpectraCell because it was the one that we had consistency with and they showed that they lengthened a little bit again. We don’t know if they lengthened all within maybe an 18-month period and they’ve stopped or if they continue to lengthen. Remember, this is only my T-lymphocytes so I can’t tell you that my whole body has been changed by it.
[Damien Blenkinsopp]: The test only looks at one specific cell.
[Liz Parrish]: Yeah. So gene therapy has a lot of obstacles that we have to get over. One thing, what genes do we need to use to create really healthy humans. The other thing is how do we target a lot of cells in the body without creating a immune response. Those are two really big things.
So a lot of people, they either go one way or another. They’re like, “This is so great that you’re doing this” or “Why isn’t this working yet?” We have a ways to go and so by analyzing this data and patients, we’re not only going to learn what happens with gene therapies, but we’re going to learn about titration. That means the dose that you give.
Here’s a really interesting thing. Hemophilia B, they just found in studies if they give 20% of the dose, they had a better outcome in patients; completely unexpected. We don’t expect that with something like telomerase induction that’s not shared outside the cells, but we can expect that with other genes. That’s cost savings. What that means to you is a lot less cost.
[Damien Blenkinsopp]: There’s a lot of those U curves in dosage. I’ve seen that talked about in other areas as well.
[Liz Parrish]: But generally in gene therapy when we look at myostatin inhibitors with the primase, the more they got, the bigger they were, but all genes are not the same.
[Damien Blenkinsopp]: Yeah. Ok. The other one you did was the MRI for the muscle?
[Liz Parrish]: Yes.
[Damien Blenkinsopp]: The myostatin?
[Liz Parrish]: The myostatin inhibitor.
(00:59:48) [Damien Blenkinsopp]: Ok. Thank you very much. Where can people follow what you’re doing, stay in touch with you? Twitter, Facebook or the company?
[Liz Parrish]: Yeah. We are in several places. Actually Brit probably knows. We’re bioviva-science.com. That’s the website. You can see what we’re doing. In October, we’re going to be offering genomic testing, but more importantly, genomic counseling because a lot of people have already got their genes run, but what does that mean?
So we want you to be able to talk to live specialists. Then we will be working over the next year to turn that into longevity counseling. We’re looking at the 59 genes in the human body that drive longevity. We want to see if in people and their family lineage, if these are actually creating longer, healthier lives by the upregulation of these proteins.
[Damien Blenkinsopp]: So they will come to you for that?
[Liz Parrish]: Yeah.
[Damien Blenkinsopp]: So you’ve got a [check 1:00:38] just like a data service basically.
[Liz Parrish]: So the genomic counseling, the genomic products, we’re hoping to offer some of the methylation testing that you can get from other companies, but offering it through our platforms so you have the availability to share your data with our company so we can solve the problem sooner. Then other than that, we’re just analyzing data and doing research and development in BioViva research and development for the larger load viral vector in order to pump you up in one treatment.
[Damien Blenkinsopp]: Ok.
[Liz Parrish]: Fifteen years give us.
(01:01:11) [Damien Blenkinsopp]: Great. If you had one ask to the audience that would help your mission, what would it be?
[Liz Parrish]: I would ask you to go and read some scientific papers. I would ask you to go look at what we’ve achieved in science, look at model organisms and how we’ve extended lifespan. I’d ask you to look at organisms that are already in the planet that have specialized genomes. The extremophiles, they can handle hot, radiation, extreme cold. Axolotls, they can regenerate their limbs. The pentachromat species that can see in billions of colours and I want you to get really excited about your future.
Our life is code and I think that we can modify that. First, we’ll look for human health and then we’ll look to enhance your life for where you want to live, who you want to be and what you want to achieve.
[Damien Blenkinsopp]: Thank you so much both of you; Brit also. Bye.
[Liz Parrish]: Thanks. Bye.
[Damien Blenkinsopp]: See you guys.
(01:02:15) [Damien Blenkinsopp]: Ok we’ve still got the lovely background noise. We’ve been running away from it, but it’s here and it’s following us so we’re just going to persevere now. So I’ve got Dr. Howard Chipman from Young Plasma with me here. We’re at RAADfest 2018. There’s basically an exhibition here. It’s an exhibition hall with lots of companies doing interesting things.
So I’m going to be talking to a selection of these people that I find more interesting and Dr. Howard is one of the more interesting people we’ve met. So first of all, could you just introduce yourself. You just gave me some great highlights of your background so I think that’s a pretty cool way to introduce yourself to they guys.
[Howard Chipman]: My name is Dr. Howard Chipman. I’m the medical director at the Atlantis Clinic in Oldsmar here in Tampa, Florida. I’ve been an emergency physician for many years and also done family practice and walk-in clinic. But I saw a lot of my patients were getting older and needed some other type of anti-aging treatments so I started doing the young plasma treatments. That’s what I’m here for to promote and also to learn about other anti-aging things that we can add to our protocols to help our patients stay alive and healthier and myself too of course.
(01:03:25) [Damien Blenkinsopp]: Yeah. So in a nutshell, what is Young Plasma and how long have you been doing it?
[Howard Chipman]: Young plasma is basically the blood minus the cells which is the plasma from younger people 16 to 25 years old. The idea is to get the healing and growth factors that you had when you were younger and replenish your body with those for anti-aging and healing of degenerative processes.
[Damien Blenkinsopp]: So you’re actually giving people basically a transfusion?
[Howard Chipman]: Yes.
(1:05:00) [Damien Blenkinsopp]: Of how much blood?
[Howard Chipman]: We customize it for the patient, but typically patients get seven units of fresh frozen plasma. The plasma comes from a certified blood bank so it’s tested for all infectious diseases.
[Damien Blenkinsopp]: This is the stuff you would get if you had an accident in a hospital?
[Howard Chipman]: Yes, this is the exact same blood you’d get in a hospital.
[Damien Blenkinsopp]: It relates to your emergency medicine background.
[Howard Chipman]: Except the donors are young.
[Damien Blenkinsopp]: So you make sure they’re young. So yesterday you were telling me that you mix up seven units of blood.
[Howard Chipman]: Actually we just start an IV and we just run the units in like an IV fluid basically over about two hours and that’s it. It’s very simple and painless.
[Damien Blenkinsopp]: Great. Well it’s very interesting. You said you’ve got a few other things just in your background.
[Howard Chipman]: Well my goal is eventually to fly into space. I love airplanes so I have a space training company also called Aurora Aerospace and we take people out for military jet training flights and also zero gravity flights. We do have a microgravity research as well as. We’ve had artists go up and do zero-G painting.
[Damien Blenkinsopp]: Cool. You’ve got an eclectic mix of interests. I like that.
[Howard Chipman]: I just can’t decide what I want to do when I grow up. With the young plasma, hopefully I won’t grow up too fast.
(01:05:08) [Damien Blenkinsopp]: Exactly. All right with the young plasma, I like to give people a little bit of background where this came from if they haven’t been aware of it. It has been in the press for the few years. So could you start from [check 1:05:19] study and then [check 1:05:20] here?
[Howard Chipman]: Well I’ll go back a little bit further. Actually, there was a Russian physician called Bogdanov in the 1920s who started giving himself transfusions of blood from young people to see if it would have an anti-aging effect. He reported many beneficial effects from it, but unfortunately he died after a transfusion.
[Damien Blenkinsopp]: He did? Of what? A bad transfusion?
[Howard Chipman]: Well they’re not sure because back then they didn’t know about blood types. So he may have had a transfusion reaction, but the patient that he got transfused from had malaria and tuberculosis.
[Damien Blenkinsopp]: Ok that could have had something to do with it.
[Howard Chipman]: An interesting note, Dr. Bogdanov was actually a communist and he was highly involved with the communists of Russia. He actually treated Lenin’s sister with young blood. So that’s the first reported instance that we know of in modern times of people using young blood or young plasma.
After that, some experiments were done where they took mice and they interconnected their circulation system called parabiosis where they took an old mouse, young mouse, stitched their blood vessels together so that their blood circulated freely between them. What they found was that the old mouse, his health improved. He became younger and basically everything they could measure or dissect out of him improved.
[Damien Blenkinsopp]: Yeah and what happened to the young mouse?
[Howard Chipman]: The young mouse, he went downhill. Other studies have corroborated this that not only is there a lack of good stuff in your old blood, but there’s actually bad stuff in there as well that actually causes bad things to you. If you take out old plasma and inject it into a younger individual, it causes damage.
[Damien Blenkinsopp]: So don’t do that guys. If you do end up in the army, if you want to ask for younger blood, i don’t know if that’s possible.
[Howard Chipman]: I don’t think so. Typically if you’re getting blood in the ER due to hypovolemic blood loss, what you really need are those red cells to provide the oxygen so that doesn’t really matter. Of course if I was dying and needed blood, I’d rather have younger blood, but if you need those red cells, it doesn’t really, that’s doesn’t matter so much.
(1:07:21) [Damien Blenkinsopp]: All right, cool. So are there any downsides to this? You’ve done this yourself.
[Howard Chipman]: Yes. I’ve been doing it for two years and I feel the difference. I feel more energetic and more youthful. I find myself acting more in ways that I did when I was younger that I had kind of forgotten.
[Damien Blenkinsopp]: How old are you?
[Howard Chipman]: Fifty-six.
[Damien Blenkinsopp]: I don’t think you look 56.
[Howard Chipman]: I used to jump up two stairs at a time. Over time, you get older and you act differently. You don’t really realize it, but after doing these treatments for a couple of years, I find myself doing things that I did when I was younger.
(1:08:00) [Damien Blenkinsopp]: Ok. What is your protocol? How frequently are you doing it? What dose?
[Howard Chipman]: I’m taking seven units every three months.
[Damien Blenkinsopp]: Ok.
[Howard Chipman]: Again, that’s not based on any hard science. It’s based on the study that we performed; the Ambrosia trial where we used seven units.
[Damien Blenkinsopp]: You are mimicking the study?
[Howard Chipman]: Yes. That dose was come upon by a high dose of plasma because we use plasma for many other things in the hospital. Basically, we just took the high upper-level dose of that and do it every three months.
(1:08:30) [Damien Blenkinsopp]: Yeah. So you said you worked on the Ambrosia study. What was the Ambrosia study?
[Howard Chipman]: The Ambrosia study was a trial where we took a number of individuals and gave them one dose of seven units of plasma and then measured the biomarkers before and after to see if there was any change in there.
[Damien Blenkinsopp]: And?
[Howard Chipman]: The study is not published yet.
[Damien Blenkinsopp]: So you’re not allowed to talk about it.
[Howard Chipman]: I don’t have the data because I was a sub investigator, but my understanding is that the amylase and the CEA showed significant improvements and there were several other biomarkers that showed that as well.
[Damien Blenkinsopp]: So reduction in amylase. Is that amylase did you say?
[Howard Chipman]: Amyloids, sorry. Amyloid.
(01:09:07) [Damien Blenkinsopp]: Amyloid plaques in the brain. How do they measure the amyloid?
[Howard Chipman]: They weren’t measuring the plaques. They were measuring blood levels. They send off a huge panel of 100 different tests.
[Damien Blenkinsopp]: Ok. Those were the only things you know that came back with a difference?
[Howard Chipman]: Correct. Correct.
[Damien Blenkinsopp]: Because you might have expected more basic things like CRP. A lot of people get elevated as age goes on.
[Howard Chipman]: It’s possible. Like I said, I haven’t seen the data yet.
[Damien Blenkinsopp]: Do you know when it’s going to be published?
[Howard Chipman: No, I don’t.
[Damien Blenkinsopp]: Ok.
[Howard Chipman]: I keep asking, but I haven’t gotten a straight answer yet. Hopefully soon.
[Damien Blenkinsopp]: Ok. We’ll look forward to that.
[Howard Chipman]: But the patients that we treated in the study and the patients I have treated subsequently have all reported subjective significant improvements in their wellbeing and health.
(01:09:50) [Damien Blenkinsopp]: Ok. Great. So now you’re providing this as a service to other people?
[Howard Chipman]: Yes.
[Damien Blenkinsopp]: By the way, are you tracking any biomarkers yourself?
[Howard Chipman]: No.
[Damien Blenkinsopp]: Have you noticed anything personally?
[Howard Chipman]: I’m not checking any real biomarkers. I do routine labs upon myself and my glucose and cholesterol and all those things improved, but it might have been due to, I started going to the gym too. I figured if I’m doing this young plasma, I might as well make the best of it and do it as a regimen of improving your lifestyle.
[Damien Blenkinsopp]: So you see improvements. That often happens with me. I do two or three things at the same time.
[Howard Chipman]: It could be from something else.
[Damien Blenkinsopp]: I don’t know, in like, ten years. So I’m going to do several and then I’m like I don’t know which one did it, but it’s something.
[Howard Chipman]: The main thing I look at is, “Does it work?” The efficacy, and I think we’re eventually going to find the cure for aging, but that’s going to be a while off. So what we need to do now is to stay alive as long as we can with the best tools that we have now. That’s what my goal is, to try and find things that we have available now that we can use to keep ourselves.
[Damien Blenkinsopp]: Extend health span.
[Howard Chipman]: Yeah extend our lifespan until maybe something better comes along.
[Damien Blenkinsopp]: Cool. You’re doing this now as a service.
[Howard Chipman]: Yes.
(1:11:02) [Damien Blenkinsopp]: How many people have you had in your clinic?
[Howard Chipman]: We’ve treated over a hundred people with this so far.
[Damien Blenkinsopp]: Are they one-time users or are they frequent? What’s the way people have been using this?
[Howard Chipman]: About half of them were in the study and they came just for one-time; some of them. But many of them have since come back. I’d say probably 80% of the people that do one treatment continue to do them because they feel improvements.
[Damien Blenkinsopp]: Are they doing the similar protocol to you? The three months?
[Howard Chipman]: Some are, some aren’t. Some can afford it.
(01:11:34) [Damien Blenkinsopp]: Let’s talk about the cost. How much is one dose?
[Howard Chipman]: The treatments are eight thousand dollars and that’s for seven units. That includes everything. If people want less units, we have a prorated scale. We have a couple patients that come every month and get five units for example. We have a patient with dementia and we’re trying to see if it can help with that. Because there are some animal models and studies that show that it might be beneficial so we’re trying to help this woman. She comes every month and we give her five units, for example.
(01:12:05) [Damien Blenkinsopp]: Ok. All right. Cool. Do you have any idea of the mechanisms? It sounds like it’s probably way off for me to understand what might be going on.
[Howard Chipman]: There are many, many things going on and we’ll never know the details exactly. Basically what we’re trying to do is reproduce the young physiology that you had when you were younger by replacing all those healing and growth factors that are present in young blood and just basically replenishing the people who are older.
There are many different mechanisms going on. The body is very complex; the process. I think over time, we’ll be able to better understand these mechanisms, but I’m not a basic research guy. I don’t have a billion-dollar lab to figure all this stuff out. So what I’m trying to do is help people today and help myself with what we have right now until we figure it out.
(01:12:53) [Damien Blenkinsopp]: Great. So one of my first questions when I met you was how are you getting this blood? Is it legal? I’m sure that might be a question some people have in their heads. So what is the answer to that?
[Howard Chipman]: Of course. It comes from a certified blood bank so yes it’s completely legal. We’ve been using plasma treatments for over 50 years in hospitals. Every hospital in every country gives plasma every day pretty much. It’s usually given as a preventative or to treat bleeding disorders. It’s an FDA-approved treatment.
[Damien Blenkinsopp]: You’re just using it off-label.
[Howard Chipman]: We’re just using it off-label for something else.
[Damien Blenkinsopp]: So it’s quite straightforward really.
[Howard Chipman]: Absolutely straightforward; no problems at all.
[Damien Blenkinsopp]: You’re just saying basically you have to be a practicing doctor.
[Howard Chipman]: Yeah, you have to be a physician because it has to be ordered and administered by a physician. There you go.
(01:13:40) [Damien Blenkinsopp]: Ok, great. So that’s Young Plasma. The other thing I would just like to know a bit more broadly what you’re up to in terms of your activities. You said you’re going to the gym and you’re tracking markers. What are you doing in terms of your own health and life extension?
[Howard Chipman]: I’m using young plasma. I’m also taking Metformin as well. It’s a Diabetes drug. There seems to be pretty good evidence now showing that it’s helpful. They did a study where they took people who are diabetics and put them on Metformin and measured their insulin, heart attacks and strokes and they actually had lower incidence than non-diabetic people who were not on the medications. I think Metformin’s a no-brainer so it’s probably a good idea to take it.
[Damien Blenkinsopp]: Did you ever raised glucose or anything like that or you’re just taking it for the longevity?
[Howard Chipman]: My hemoglobin A1C was measured at the time was borderline. It wasn’t diabetic.
[Damien Blenkinsopp]: Was it six?
[Howard Chipman]: It was 5.7. I used to joke and tell people I was one doughnut away from being borderline, but it’s all back to normal now.
[Damien Blenkinsopp]: Where’s it at?
[Howard Chipman]: I don’t remember what it was last, but it dropped. It dropped. It was almost in the abnormal range and now it’s well in the normal range.
[Damien Blenkinsopp]: So do you think that might be the Metformin?
[Howard Chipman]: I tested it before I started the Metformin so I just started. I haven’t checked my blood. I just started on the Metformin recently.
[Damien Blenkinsopp]: It’s probably the young plasma and your exercise.
[Howard Chipman]: Yes. Yes, but the Metformin will bring it even lower. Sorry, what were you asking about?
[Damien Blenkinsopp]: I don’t know. It skipped my mind there. This is the problem with live. I got into what you were talking about.
[Howard Chipman]: You asked me what I do for other treatments. So firstly, I take Metformin, I do the young plasma, take an aspirin a day; that’s a no-brainer.
[Damien Blenkinsopp]: Ok aspirin.
[Howard Chipman]: I also take cholesterol medication. I take a statin.
(01:15:31) [Damien Blenkinsopp]: Are you concerned about the potential negatives of some of those?
[Howard Chipman]: I don’t see statins as a problem. It’s overblown. A lot of my patients are 400 pounds, their cholesterol are through the roof, “Oh they can’t take a statin.” I do not see many problems with statins. Rarely, people get some muscle pain and you have to stop it.
[Damien Blenkinsopp]: Like fibromyalgia.
[Howard Chipman]: In some people, it will raise their liver enzymes slightly.
[Damien Blenkinsopp]: The things I have seen are its potential interactions with mitochondria. I was thinking that might be the connection with the muscle pain and fibromyalgia.
[Howard Chipman]: It’s possible.
[Damien Blenkinsopp]: The connection there.
[Howard Chipman]: But I don’t see too many side effects from it. Most people don’t have any problems at all. So I take that because my cholesterol was a little bit high. There are studies suggesting that even normal people take statins significantly to reduce their risk of heart attacks and strokes. My father had coronary artery disease.
[Damien Blenkinsopp]: You’re focused on that one.
[Howard Chipman]: Yeah. My dad didn’t believe in eating vegetables. He lived to be 90.
[Damien Blenkinsopp]: He would’ve gotten along with, have you heard of th carnivores? The zero-carb? There’s a whole tribe of them on the internet now.
[Howard Chipman]: Really?
[Damien Blenkinsopp]: They just eat pure meat. That’s a thing, yeah. Great. So you’re exercising, you’re taking Metformin, baby aspirin; you’re doing quite a range of things.
[Howard Chipman]: And the statin.
[Damien Blenkinsopp]: And the statin, yeah. That’s quite a bit.
[Howard Chipman]: The other thing I’m looking into is Rapamycin as well. I’ve seen some potentially good studies and evidence on that. It is an immunosuppressant, but some studies show if you take it once a week, you don’t get the immunosuppression, but you still get the anti-aging effects. I have a couple of my young plasma patients that have dementia. I have them on Rapamycin.
[Damien Blenkinsopp]: Is it quite expensive?
[Howard Chipman]: It’s not cheap and it’s not very expensive either. You’re only taking it once a week.
[Damien Blenkinsopp]: How much does it cost on a monthly basis, for example?
[Howard Chipman]: Can I just throw a number out like 50 bucks, 100 bucks. It’s not cheap.
[Damien Blenkinsopp]: That’s pretty cheap.
[Howard Chipman]: I thought a four dollar Wal-mart prescription, but it’s not expensive. It’s not expensive. It has been out for a while. That’s something I’m not taking, but may consider taking soon because it looks like it does actually work.
(01:17:43) [Damien Blenkinsopp]: What will lead you to the decision to take that or not?
[Howard Chipman]: Maybe I’ll see how my patients do on it.
[Damien Blenkinsopp]: Ok guinea pig; the guinea pig approach.
[Howard Chipman]: Usually, I use myself as the first guinea pig.
[Damien Blenkinsopp]: That’s good to know. It has been great to chat with you about all of this. Is there anything we missed?
[Howard Chipman]: Not that I can think of. I think you asked me to give my contact information. Anybody has any questions, they can contact me at any time. I’m at the Atlantis Clinic in Oldsmar, Florida. That’s next to Tampa. Our website is young-plasma.com and if anybody wants to call me for a consultation, I’ll give you my cellphone number 813-476-2321. If you have any questions about Young Plasma or any other anti-aging, I’m glad to answer for you.
[Damien Blenkinsopp]: Thank you so much for your time. It has been great to have you here.
[Howard Chipman]: Nice talking with you.
(01:18:37) [Damien Blenkinsopp]: Hey! We’re here with our second interview. There’s a little segway here actually. We happen to have one of the guys who’s using
[Brian M. Delaney]: Young Plasma.
[Damien Blenkinsopp]: Young Plasma which I didn’t know.
[Brian M. Delaney]: From Dr. Howard Chipman. I got that six, seven, eight weeks ago and I didn’t know what to expect. I read some of the research results. There are actually many and there’s lot for umbilical cord plasma which is really young plasma, but for less young plasma, there aren’t a lot of results out there, but I wanted results. For theoretical reasons, I expect there to be some benefit because I’m 55 and the plasma comes from someone between the ages of 16 and 25.
I did some before and after biomarkers and saw small changes, but it’s hard to know because I’m always changing my diet and exercise routine so I can’t really say much about that. What was amazing was the subjective effect which sadly didn’t last too long, but for about 36 hours I was Superman. It was amazing.
(01:19:35) [Damien Blenkinsopp]: What did it feel like to be Superman?
[Brian M. Delaney]: I have sleep problems and I’m not as young as I used to be. I think I do have a lot of energy and I’m in pretty good shape, but I walked towards my car from the clinic after having plasma. During it I had, some get hives so I had some Benadryl so I was a little tired from the Benadryl, but that had worn off. I got in my car, turned on the radio and the music sounded more beautiful. It didn’t matter if it was Abba or Beethoven, the whole thing from the bass.
[Damien Blenkinsopp]: Life is more beautiful.
[Brian M. Delaney]: Yeah, it was amazing.
[Damien Blenkinsopp]: There were more colours in the world.
[Brian M. Delaney]: Yeah it was incredible. I’m driving across the Everglades and it’s just wow.
[Damien Blenkinsopp]: It was a bit psychedelic.
[Brian M. Delaney]: It was almost. I happen to be a birdwatcher. You can fool yourself into imagining and experiencing it better than it is. So I’m looking at all these passing raptors and identifying them really quickly as if my vision worked better. I knew obviously my vision is not better. Anyway so for about a day and a half, I really felt physically, I felt, you could even say I had more energy. That’s such a stupid marketing term, but I really did have more energy.
I slept better that night which is unusual for me. Normally I have to take sleep medications which is not good. The next day I woke up and I felt amazing. I did, this is one slightly more objective measure, I do decline pushups and I changed my diet and I tried to see if it would have an effect so I measured the height of my feet on the chair exactly, it’s 47 centimeters, arms are set a certain distance apart and I could do about 15% more that morning.
[Damien Blenkinsopp]: So how many?
[Brian M. Delaney]: Normally, it would be about low forties and it was somewhere around 50 I think.
[Damien Blenkinsopp]: It pushed you to the maximum?
[Brian M. Delaney]: That was just maximum, yeah. Next day, I was exhausted, yeah. Unfortunately, the subjective effects and partially objective.
[Damien Blenkinsopp]: Did they decline as well? That change during the week?.
[Brian M. Delaney]: It did start to go back to normal after about a week. So the 36 hours was just an amazing experience and then it started to fade and within five to seven days, I felt like I was back to normal.
(1:21:42) [Damien Blenkinsopp]: So when did you do that?
[Brian M. Delaney]: I can’t remember exactly. I think it was maybe two months ago. It was seven weeks.
[Damien Blenkinsopp]: You just did it once?
[Brian M. Delaney]: Just once although I’m going to do it again in a couple of weeks.
(01:21:50) [Damien Blenkinsopp]: So do you have a plan? Are you going to stick to it?
[Brian M. Delaney]: Here’s what I’m going to say. Money is an object for me, but if money were no object, I felt so good that I would do this every three or four days. That’s how good I felt, but it’s just too expensive. Dr. Chipman knows that and he would love to bring the cost down. He has a contract with the blood bank which is hard to get that enables him to buy small quantities of plasma.
[Damien Blenkinsopp]: I think he’s going to be limited. He was telling me it’s quite tricky at that place.
[Brian M. Delaney]: Yeah so I would love to do it every few days. That’s how good it felt, but it’s just impractical.
(1:22:37) [Damien Blenkinsopp]: Ok. Now Brian M. Delaney, let’s introduce you. Who are you? What do you do?
[Brian M. Delaney]: I am currently the president for the Society for Age Reversal. It’s a group that Bill Faloon founded.
[Damien Blenkinsopp]: Bill?
[Brian M. Delaney]: Bill Faloon of Life Extension.
[Damien Blenkinsopp]: One of the founders of Life Extension Foundation or supplement maker?
[Brian M. Delaney]: Exactly. He put me in charge of it. Lots of people, fortunately more and more all the time, are working on finding cures for aging or at least treatments to reverse parts of aging. It’s great that lots of money is coming in from increasingly conventional sources.
For example, Jim Mellon, the British millionaire was a very good, but more or less conventional investor. He slowly started turning towards Biology and then now he’s turning towards anti-aging. I’m sure it’s probably because he has charitable donations and he wants to save himself and his immediate family, but also because he has realized it’s a great investment.
So lots of money is going into anti-aging, but typically this is going to result in cures or effective treatments maybe a decade from now. The typical drug development path takes that long; maybe seven years, maybe fifteen years. What we’re trying to do is find what one could describe as the low-hanging fruit of age-reversal treatments. That’s not entirely accurate, it’s just easy to pluck. It’s not always easy to pluck, but you can pluck it soon.
So this involves things that have been investigationally orphaned because there’s no easy way to make a profit from it. For example, Metformin that has been studied for a long time for Diabetes, but now there are people trying to raise money during these trials to try to test it in humans as an anti-aging treatment, but how do you make a profit from a drug like Metformin? It’s not so easy. You can do it as a clinician, but that’s just patient fees so it’s not going to be too profitable.
[Damien Blenkinsopp]: [Check 1:24:39].
[Brian M. Delaney]: Yeah. Rapamycin is another example and of course senolytics. Senolytics are substances that will destroy senescent cells; these zombie cells that spew out injurious cyclin molecules.
[Damien Blenkinsopp]: The idea is we accumulate senescent cells as we age and it’s the signals they’re sending out or the metabolites or whatever they’re sending out which is damaging and accumulates over time.
[Brian M. Delaney]: That’s exactly right. Worse still, these senescent cells can turn other non-senescent cells into the senescent cells. So it almost is like The Walking Dead. So a TV show where zombies can turn non-zombies into zombies by just being near them and getting close and biting them metaphorically speaking. So it’s great tool to use if you can do it safely. Some would say that’s a big if. The category of senolytics spans both the traditional [check 1:25:37].
[Damien Blenkinsopp]: So senolytics are things that kill senescent cells?
[Brian M. Delaney]: Yeah. “Seno-” from the Greek “old” and “lytic” for “lysis” to split apart or break so yeah that’s what senolytics do. There are all kinds of them.
[Damien Blenkinsopp]: Are these compounds or molecules?
[Brian M. Delaney]: Yeah. Even now, there are new strategies using enzymes, but the standard approach that has existed upon not only Big Pharma, but also the stuff that we’re trying to find, involves either something like natural substances like fisetin or old cancer drugs that can be repurposed like Dasatinib.
So that has been tested in rodents several times now specifically a combination of Dasatinib and Quercetin. Synergistic is a word that is often abused, but it describes them correctly. You put them together and the effect is more than the sum of the individual effects of the two. I don’t think there has been a lifespan study done yet or even underway, but what we see in the rodents is regression of atherosclerotic plaques. for example.
[Damien Blenkinsopp]: Regression?
[Brian M. Delaney]: Yeah, actual regression which is astonishing which we normally think they can’t do. Dean Ornish I think has shown that a radically low-fat diet combined with other aspects of his program, meditation and exercise can regress them actually, but aside from that, it’s really hard.
[Damien Blenkinsopp]: How do they measure that?
[Brian M. Delaney]: I think it was just x-rays.
[Damien Blenkinsopp]: [Check 1:27:09]?
[Brian M. Delaney]: With the rodents, I think they actually just looked. They just x-rayed them, I think. I’m not sure. There are actually two studies I believe that showed that.
(1:27:19) [Damien Blenkinsopp]: So when you say you going about looking for these compounds, what does that actually mean? Are you looking for the research? Are you talking to people?
[Brian M. Delaney]: We’re talking about senolytics alone, but this is the same strategy for lots of other drugs.
[Damien Blenkinsopp]: You’re doing several areas. This is just one you’re focusing on at the moment?
[Brian M. Delaney]: I’m focusing on many, but it’s one that I’m particularly interested in. So I think we can actually save people’s lives now with senolytics. I’m convinced. I’m trying to get my mother to try this and she’s a little scared because Dasatinib is a cancer drug and if you Google it, you see the side effects. That’s from people taking it daily for months who are really sick because they have cancer and are taking other drugs.
My approach partly is I just read research. My formal academic training is in the Humanities, but I’ve gotten up to speed as fast as I can on research. I try to make executive decisions about what areas our group needs to focus on and then I contact the real experts which I’m not, and try to form collaborations and try to see if what they’re doing in researching Quercetin alone or in combination with something else is redundant. Then we try to find funding. We might fund it ourselves. Bill Faloon has funded lots of projects; he’s incredibly generous. And or we find other people who want to fund some of this research.
At conferences, the talks are always great, but you go to the poster presentations and you find some mad scientist graduate student at the University of Lund in Sweden. He has got some cool idea and it may be something that hasn’t even been published yet. That’s what I really want to do. I want to find these things that no one knows about.
(1:29:10) [Damien Blenkinsopp]: Yeah. So just for the people out there, posters at conferences are typically studies in progress or maybe just finished by PhD students. Maybe it’s part of their PhD so they’re not going to do a full talk on it, but they’ll have this poster explaining that whole study and what they found or they’re finding. So I actually have PhDs working for me who present this kind of stuff at conferences so it’s a way to fund the edgier, earlier stuff.
[Brian M. Delaney]: Exactly. Yeah. Then there are small startups or we call them pre-startups. Scientists with ideas who are sitting somewhere. There’s a guy Harold Catcher who’s actually an American, but he’s got a collaboration with some people in Mumbai and actually he’s spent about half a year.
At this point, unfortunately, I can’t talk about much of his research, but they have a polyherbal formulation that has got some amazing results. It can look like it does what this calorie-restriction diet does which apparently slows aging even in humans according to biomarkers. That research has been done for a century. They have some other amazing products and they’re forming a company.
[Damien Blenkinsopp]: So it’s like a calorie restriction mimetic?
[Brian M. Delaney]: Yeah, possibly even more. It’s not clear yet. So people like that I try to identify and then maybe that would be a case where if they’re forming a startup and they’re looking for investors as opposed to funding from charitable sources that just want to give away their money to help resources then I might connect them with investors who want to help actually found the company. If the company’s already started then I’ll help it grow.
(01:30:57) [Damien Blenkinsopp]: So your goal is basically to find opportunities and help push them on?
[Brian M. Delaney]: Yes.
[Damien Blenkinsopp]: You help give them what they need to grow and to make more progress faster?
[Brian M. Delaney]: Faster! That’s really the key. If you don’t have that part about the timescale, you’re really focused on the short-term. We had a group that was named previously “The Society for the Rescue of Our Elders.” A long and exotic name, but the concept was it was based on this group, the name itself, this group in Holland that existed two and a half centuries ago where people would fall into the canals and drown. If you did it quickly enough, you could pull them out and save them so it was a society for the rescue of drowned persons.
The idea was there are people like my parents who are about 83 who don’t have a lot of time left. My dad’s in good shape, but my mother is not. Jim Mellon is doing this amazing work, Juvenescence, but a lot of what he’s investing in is not going to come in time for my mother; probably not even my father who is still in good shape. So the idea with this society for the rescue of our elders, I thought of it internally as the society for the rescue of my mother. That is what really motivated me.
[Damien Blenkinsopp]: That gets you up in the morning.
[Brian M. Delaney]: Exactly. So it’s really trying to find treatments that can be made available in a very, very short timeframe. If you saw my mother sitting down, she’s sharp, but if you saw her walk, you’d realize she may not have a lot of time left. It actually does worry me so that’s part of why. I had a fine life teaching Philosophy in Sweden. I gave that up entirely to work with Bill because I really want to.
(1:32:43) [Damien Blenkinsopp]: Wow! I’m just really curious. How did you get involved in this?
[Brian M. Delaney]: Life Extension itself, I was involved in the Calorie Restriction Society and that goes way back.
[Damien Blenkinsopp]: I saw that, yeah.
[Brian M. Delaney]: I was making money doing other things. I was in graduate school as a philosopher.
(01:32:59) [Damien Blenkinsopp]: So you practiced calorie restriction?
[Brian M. Delaney]: For a long time. I’ve gone at least half of it which I’ll explain in a moment. What happened was a long time ago in 1992, I was diagnosed with Crohn’s Disease. It’s an inflammatory bowel disease. It was not clear at first. We thought it might have been food poisoning, but until that point, I really ate horribly. I exercised a lot. I had this notion like a lot of people do that the virtue of exercise can make up for the vice of bad eating no matter how badly you eat and that’s not true of course. It helps to exercise, but you have to eat well as well.
Back then, research online was useless so I went to the medical library when I was in graduate school in Phiolosophy, but I would go to the medical school and read about nutrition. That’s when I found what [check 1:33:45] were and calorie restriction. I called them up and said, “This looks miraculous! Why aren’t human beings trying this?” They said well I’ve written two books trying to get people to do it. A few people are, but let’s start a non-profit. That was my beginnings of my interest at Life Extension.
But back then, because I was so focused on things one can do now, then as now, and then it was only CLR. Vitamins couldn’t help with certain disease states, with aging cells so CLR was the only thing I wanted to do. So I did that, but meanwhile I’m in graduate school. That was my main way to make money; not much.
Then I accidentally moved to Sweden 18 years ago and continued making money teaching all the while trying to keep the CR Society going. But what happened about seven, eight, nine years ago was there really were better options or options other than CR (Calorie Restriction) that seemed promising; that seemed either available or soon to be available.
So that posed two challenges for me. One, do I even want to keep the CR Society going given it’s clear [unclear 1:34:53]?
[Damien Blenkinsopp]: It has less potential.
[Brian M. Delaney]: Exactly. But then secondly, do I want to shift gears and put more of my own energy into something else? So I oscillated for quite a while and then just by chance, I was in Florida a year ago only visiting my parents and helping them move actually and called up Bill Faloon thinking that I might maybe write an article for a magazine about CLR. I think what I wanted to pitch was, “Is it still worth it?” He said, “Where are you?” I said I’m in Florida. “Hey I’m in Florida, let’s have dinner.” We had dinner and we talked. We had another dinner and we talked.
He had already started this Society for the Rescue of Our Elders. He said if you want to become Project Manager, leave your life in Sweden and just really commit to this, I’ll bring you on retainer and we’ll be off and running and I said yes.
[Damien Blenkinsopp]: Excellent! I bet you were like man, this will be fun.
[Brian M. Delaney]: It was generous of Bill and great for me. Not that I minded teaching Philosophy to hungover Vikings.
[Damien Blenkinsopp]: So remind me. Is this now two years? How long?
[Brian M. Delaney]: One year.
[Damien Blenkinsopp]: One year.
[Brian M. Delaney]: A little bit more.
[Damien Blenkinsopp]: Where are you at with this? Are you basically working some leads or have you actually completed some funding?
[Brian M. Delaney]: Where we are now is what’s going to be announced here at RAADfest by Bill Faloon in a few hours and then in a little more detail in his second presentation on Sunday which is that we now have a pretty good idea of some concrete steps people can take today to slow aging.
[Damien Blenkinsopp]: This is under senolytics?
[Brian M. Delaney]: It involves a number of steps. I feel like I don’t want to go into it in too much detail because Bill wants to.
[Damien Blenkinsopp]: Yeah, open it to the world.
[Brian M. Delaney]: Yeah, be the one to present it. We have a little publication that you can grab where it’s laid out. None of this has been verified and done in phase three or even phase two trials. This is just stuff that we have either put together using other people’s research that others have funded or research that we have helped fund through this group called Better Humans. This guy, James Clement started a non-profit, Better Humans where he runs these open-label, non-randomized simple controlled trials. They call them phase zero trials; exploratory trials. So some of the data from his work.
[Damien Blenkinsopp]: So it’s on humans, but it’s non-randomized. So you basically just give ten people something and see what happens?
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: You take a baseline?
[Brian M. Delaney]: Exactly. In some cases, one can say well that doesn’t really say that much, but in this case he designs them very well. Give me a moment, I have to remember what I’m allowed to say. You know what? Wait until Bill gives his talk. I don’t want to screw this up. James doesn’t really care, but it’s all pre-publication and he has a whole bunch of papers that are about to be accepted I assume for publication. I am allowed to say that the results from most if not everything he has done look positive in two ways. They’re safe and at least a bit efficacious.
(1:38:07) [Damien Blenkinsopp]: So what could this mean? Would it mean there’s a supplement someone can take with these compounds?
[Brian M. Delaney]: It will mean yes supplement or drug in a particular order. Actually I should just back up. This has nothing to do with Better Humans.
[Damien Blenkinsopp]: This is a senolytics area?
[Brian M. Delaney]: That is part of it, but something that is important here to back up and note. It has nothing to do with Better Humans or any research that we’ve done recently, but it’s almost common sense. I’m going to to express broadly that the most fundamental first step that people should do is to get the body in basic shape using things like Vitamin D supplementation if your Vitamin D is too low or get out in the sun, exercise if you’re overweight, eat better. These things are actually more effective than a lot of people realize.
I’m still President of the CR Society and I still want to wear that hat occasionally and tell people even if they don’t want to do extreme CR like I did for years, that can help a lot. Then take these steps that involve some of these off-path drugs.
(1:39:16) [Damien Blenkinsopp]: So build your foundation first with the basics that we know. All right let’s talk about the structure though because that’s interesting and maybe it relates to what you do. I don’t know.
[Brian M. Delaney]: For my own personal health?
[Damien Blenkinsopp]: Have you implemented all of this stuff already?
[Brian M. Delaney]: Absolutely!
[Damien Blenkinsopp]: So let’s just talk about you as a case study. So what do you do?
[Brian M. Delaney]: What I did for a long time was calorie restriction as you know.
[Damien Blenkinsopp]: How many calories we’re talking per day?
[Brian M. Delaney]: I’ve got weird inefficient metabolism. This is going to sound like a lot, but I exercised a lot. At my most extreme, where I really looked like I shouldn’t have survived; it was really extreme.
[Damien Blenkinsopp]: Very thin.
[Brian M. Delaney]: Very thin. I looked in the mirror and I thought that’s not me even though I felt great. At that point, I exercised a lot. I was eating 1,900 calories per day and that doesn’t sound so little.
[Damien Blenkinsopp]: What is it? Like 10% now?
[Brian M. Delaney]: No, it was more like 35% to 40% below what I’m eating now. I’m still trim, but not like [unclear 1:40:18].
[Damien Blenkinsopp]: You’ve got quite a high metabolic rate.
[Brian M. Delaney]: Yeah which is actually bad because that tends to be one of the things correlated with rapid aging. It’s just burning through like stepping on a gas pedal and the engine is not quite in tune so that’s unfortunate. Anyway I did that for a long time and looked at my biomarkers which improved dramatically.
[Damien Blenkinsopp]: What kinds?
[Brian M. Delaney]: Just like HDL through the roof, LDL really bottom down. When I did the measuring particle count and size, the few LDL particles I had weren’t noticed.
[Damien Blenkinsopp]: Great. So that’s what you want basically. You probably don’t remember the numbers.
[Brian M. Delaney]: No.
(1:40:57) [Damien Blenkinsopp]: It would be well below 800 for the small particles. But based on what you’re saying well below 800 so really good. We’re talking about the nuclear magnetic resonance lipoprofile which is a test which looks at the particle size of your LDL and your HDL to really understand that versus just looking at LDL cholesterol total which is normally what people look at. The idea is that it’s a lot more accurate because if you’re looking at total LDL, you could have some really big particles which we don’t really care about because they’re not very atherosclerotic.
[Brian M. Delaney]: We think.
[Damien Blenkinsopp]: We think. It’s a better assumption than LDL is bad for you.
[Brian M. Delaney]: Absolutely.
[Damien Blenkinsopp]: It’s progressing slowly is what we’ll say. But if you combine that with a bunch of biomarkers then it starts to paint a realistic picture. So your homocysteine, your CRP, did you look at those?
[Brian M. Delaney]: Yeah. CRP was just perfect; it couldn’t be better. I do have genetically high homocysteine so I didn’t get below seven. Seven is very good.
[Damien Blenkinsopp]: Seven is actually good.
[Brian M. Delaney]: It’s good, but I a lot of people have below five. I have familial high blood pressure so mine never got without having orthostatic hypertension which is fainting when they stand up. They would have 85/57 and feel great. Mine was more like 102/60 which is great, but it’s not the typical extreme CR value. My fasting glucose was, my doctor would say, “Do you feel weak?”
[Damien Blenkinsopp]: How much was it?
[Brian M. Delaney]: It was like 60; usually sometimes even high fifties.
[Damien Blenkinsopp]: Yeah, that’s pretty low.
[Brian M. Delaney]: So it was great. I felt great. Unfortunately what happened was about three years ago, two and a half years ago I had hernia surgery and they screwed up so then it was three surgeries. I had to eat more to recover. You have to eat more. I don’t know if it had to do with mTOR signaling, but I had to get out of the famine stage which doesn’t make growth easy. But I have to confess when I started eating more, I felt good in a way that made me think wow.
[Damien Blenkinsopp]: Alive! Just some empty calories after all.
[Brian M. Delaney]: Leucine, the protein that makes the mTOR signaling go up and testosterone. Suddenly, I was a man again.
[Damien Blenkinsopp]: Did you test your testosterone? Because I thought it would go down while you’re fasting.
[Brian M. Delaney]: It did.
[Damien Blenkinsopp]: Which is similar to caloric restriction I would think.
[Brian M. Delaney]: It’s two things. People on really extreme CR have low serum total testosterone, but really low free testosterone because the sexual hormone binding is really high.
[Damien Blenkinsopp]: It does?
[Brian M. Delaney]: Yeah. On CR, that took place. We joke that men on CR, we are functional eunuchs. When I started eating more, I realized that there is perhaps more of a sacrifice to being on CR than I realized. Being hungry was not a problem for me. Feeling cold is not a problem; you put on a sweater.
[Damien Blenkinsopp]: You were starting to realize also that CR may not be as impactful compared to all these other things.
[Brian M. Delaney]: That too.
[Damien Blenkinsopp]: So you got double whammy.
[Brian M. Delaney]: Yeah. Exactly. So that got me thinking about alternatives. At that point, I started, well I had to recover from my surgeries. That took a while. So then I started going back to research in my off hours and then that’s when I started to realize how much else is out there. I looked into Rapamycin and some new [check 1:44:21] that appears to be a partial calorie restriction that I’m now on by the way.
[Damien Blenkinsopp]: You’re on Rapamycin?
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: Let’s get your stacks.
[Brian M. Delaney]: So to answer your question, I got off CR, had a bunch of testosterone and had fun with that. Then I realized ok I had to get serious about not dying.
[Damien Blenkinsopp]: How old are you by the way?
[Brian M. Delaney]: Fifty-five.
[Damien Blenkinsopp]: Fifty-five.
[Brian M. Delaney]: I was more knowledgeable about diet than anything else. What I started with was time-restricted eating. I didn’t want to go back on CR the way that I had been. I wanted some of the benefits of a CR-like diet so I was interested in Valter Longo’s work and I tried the Fasting Mimicking Diet for a while.
[Damien Blenkinsopp]: How many cycles did you do?
[Brian M. Delaney]: I did it once already for five or six weeks for about four months. It’s hard to know. You get these immediate benefits after and then they start to fade. It’s not clear. He hasn’t done the experiment which is really important which is to do daily CR with an amount that is the average amount that someone on fasting would have done would end up eating. If you eat 2,500 calories per day normally and then you ate 500 per day for five days of every month. You average that out and what is that per day for the month?
Then you do the study with normal daily CR eating the same total amount averaged over the month. He hasn’t done that.
[Damien Blenkinsopp]: So you want to compare?
[Brian M. Delaney]: Because we don’t know if it’s fasting per se.
[Damien Blenkinsopp]: You’re saying it might just be the calorie reduction because five days you reduce your calories?
[Brian M. Delaney]: We don’t know that. He needs to do the experiment.
[Damien Blenkinsopp]: I’ve done an experiment with fasting [check 1:46:02] last week. Your immune system goes down. It’s going to go down further because there’s more autophagy than with caloric restriction.
[Brian M. Delaney]: We don’t know that.
[Damien Blenkinsopp]: No?
[Brian M. Delaney]: We don’t know that actually. With mild CR, maybe not. But certainly daily CR, at least moderate CR, there is autophagy. There are all kinds of things that happen at slower levels than during a fast or a fasting on your diet.
[Damien Blenkinsopp]: What I do know is just your white blood cell count is halved on day five. Then you test again seven days later after refeeding and you’ll actually be higher than your baseline and that’s what I’ve seen several times now.
[Brian M. Delaney]: In yourself?
(01:46:43) [Damien Blenkinsopp]: Yeah. So I’m using as a proxy for autophagy which isn’t great, but it’s difficult to get autophagy and get a biomarker. So I’m continuing to look into that, but it gets me hopeful. I’ve also seen some effects in my mother who is now doing cycles of these to combat cancer. It looks promising from that. For her type of cancer, you have immunoglobulin M which grows over time. So the maximum reference [check 1:47:10] because basically over the years, it has grown.
What we’re trying to do is knock it down by doing a fasting mimicking diet once a month. We’ve seen it now two times in a row now boom boom. That suggests to me autophagy because that’s the idea behind why I wanted to implement it with her is that she’s getting that autophagy, it’s clearing up some of the senescent, well cancer cells in this case, not just senescent, but evil cancer cells. We’re hopefully replacing with some of the good cells. But I understand it’s hard to get that autophagy that’s actually going on.
[Brian M. Delaney]: I was trying to figure out do I want to keep doing this? I was confident I didn’t want to do daily CR because that was just horrible.
[Damien Blenkinsopp]: It seems like you’re taking some of the fun out of life. Your testosterones are always going to be low.
[Brian M. Delaney]: Although what I argued before I experienced this surge of testosterone after my surgery which doesn’t matter because I was able to have normal relations with women and I was able to fall in love.
[Damien Blenkinsopp]: For the first time in my life.
<b[Brian M. Delaney]: No I mean before.
[Damien Blenkinsopp]: Ok. No. Good.
[Brian M. Delaney]: Finally, at the age of 52, I fell in love and had sex.
[Damien Blenkinsopp]: I realized why.
[Brian M. Delaney]: Exactly. But on the other hand, love does have a component that is obviously physiological and a lot of it is lost. What we told ourselves was that we have a more sublime form of love like what Socrates describes in Plato’s Symposium. The character Socrates; I don’t know if everyone knows that. You start with the body and then Diotima is the character that Socrates himself talks about saying that we become more sublime as we love in a less corporeal way. We had all these notions of how we were in some ways still able to love and it was better. That’s absurd. It has to be sexual and plunge more directly.
Anyway so I knew I didn’t want to do daily CR. I experimented with it fasting mimicking diet before. I may still do that periodically. It’s not something you have to choose one way of doing and stick to it. Then I tried this restricted eating window daily, but that was too difficult because I exercised.
[Damien Blenkinsopp]: Which hours?
[Brian M. Delaney]: This is the problem. This very controversial, but there is some evidence to suggest that we do need to eat our first meal not too late in the day. That’s controversial.
[Damien Blenkinsopp]: Actually I have seen such in Panda’s work. He’s really pushing that we shouldn’t be eating late in the day. I have been using that template since seeing his work.
[Brian M. Delaney]: Now there’s also this, there’s a lot to work on, which genes turn on and off in the normal circadian cycle. A lot of it based on work with rodents which are nocturnal so it’s hard to know if you can flip that to the diurnal pattern for humans, but it seems clear that there are changes in genes. We don’t know in humans what they are, but there are these go, have sex during the day.
[Damien Blenkinsopp]: What you’re saying is we have a genetic clock basically? The circadian clock.
[Brian M. Delaney]: Then at night, you get into this repair mode that could be interfered with if you have a belly full of food. This is why we’re all different and we can’t come up with general rules that everybody follows. My problem, and some other people have this, is that I have horrible sleep problems. They got worse around seven or eight years ago. That’s another reason why I had to go off CR. Somehow the low blood glucose at night was causing an increased cortisol.
[Damien Blenkinsopp]: You get a cortisol spike, yeah.
[Brian M. Delaney]: Maybe that was happening all along and I became more sensitive because I got older or maybe the spike went higher. I don’t know, but something changed. That’s another reason why I just cannot be on CR unless I’m going to take really powerful probably brain-damaging sleep medications which I don’t want to do.
[Damien Blenkinsopp]: It defeats the purpose of the whole thing.
[Brian M. Delaney]: Yeah so I make it 80 and I’m just drooling and I don’t know my name unless I’m [check 1:51:16] before I become drooling. At first, I tried a time window that was late because of my sleep problems and I just was too scared that I’m screwing up this cycle of genetic changes. So then I tried an early window, but then I couldn’t sleep.
I was trying to find some safer sleep medications than the so-called “Z” drugs. They have these non-Z like names such as Ambien which is zolpidem, but there is one with a short half-life called Zaleplon which I think is Sonata. I always forget the easy to remember names. I think it’s Sonata that I would take because I have sleep maintenance insomnia. My head won’t go to sleep and I wake up after four hours and I can’t go back to sleep so I’ll take Zaleplon then, but that’s still not so safe so I gave up on that because I woke up too early.
[Damien Blenkinsopp]: So do you wake up early?
[Brian M. Delaney]: Yeah, I wake up too early and I can’t get back to sleep.
(1:52:06) [Damien Blenkinsopp]: I have problems with that too.
[Brian M. Delaney]: It’s horrible unless I stuff my face before I go to bed.
[Damien Blenkinsopp]: Have you tried CBD oil?
[Brian M. Delaney]: Yeah. I haven’t found really pure CBD oil is my problem.
[Damien Blenkinsopp]: I think that might be part of the problem. I managed to get a whole bottle from this person I knew and it did seem to help, but only if I took it once in a while. If I start taking it every night, it stops working. It doesn’t do the trick.
[Brian M. Delaney]: All right, but that’s a whole other topic; how to manage sleep. What I’m now doing is I’m suffering the different types of damage. I cycle through different things that are useful, but damaging in different ways. So I’ll eat late a couple times a week then I might get reflux which is another problem and I might screw up the genes that are supposed to turn on. That’s only couple times a week.
I’ll take the CBD oil. The reason I asked about the purity is not so much about the strength, but typically there will be a little bit of THC mixed in even though it’s illegal. I need a huge amount of CBD to have an effect, but this means a huge amount of THC. So I do that a couple times a week and wake up half-stoned I think. Maybe it’s the CBD that’s making me feel that way. Then a couple times a week, I’ll get [unclear 1:53:17].
[Damien Blenkinsopp]: It feels like it if I take more. I’m quite sensitive to it. I don’t need a lot.
[Brian M. Delaney]: You’re lucky.
[Damien Blenkinsopp]: I’ll wake up really drowsy in the morning and I need two coffees to wake up. It avoids the purpose.
[Brian M. Delaney]: I’ve tried this Suvorexant from Belsomra which works on this new system discovered; the orexin receptors. It works, but it feels like it has a long half-life.
[Damien Blenkinsopp]: I don’t know if this would be helpful, but one of the things that helped me a lot is I found a Parkinson’s study because I have the same night-waking problem. They did this experiment where basically they gave them a strong light source, 10,000 watts SAD lamps; the Seasonal Affective Disorder lamps, that are medical lamps. You put one of those in front of you and you expose yourself to that for an hour in the morning. I think it was actually half an hour, it wasn’t that long, but I’ll leave mine on for an hour sometimes.
I bought one of these and just put it next to my laptop when I’m working in the morning and you’re just given that stronger relative signal because we don’t get outside. I’m in London and it’s terrible. You actually don’t even know if it’s daytime sometimes when you look out the window.
[Brian M. Delaney]: It’s like that in Stockholm.
[Damien Blenkinsopp]: Whereas here we don’t have that problem at all. That has seemed to help. I do it every morning because it’s right next to my computer.
I knew it was working because at first what I tend to do is have my coffee and then I’ll feel alive and I would switch it on and just check the usual business stuff. Has anything blown up while I was asleep. So I’ll do that and really wanted to have my coffee, but I actually don’t need it. I’m already really awake. So I started to notice that and then on the other end of the spectrum, I was getting more sleepy in the evenings because now you’ve increased the relative distance. You have a strong light in the morning and now when it gets dark in the evening, I was starting to feel drugged and I’d start going to sleep at 9 o’ clock, no problem.
Then the other thing that has really made a difference is getting to bed earlier. If I can get to bed at 9:00, I’ll still wake up at 4:00, but I’ve had seven hours of sleep.
[Brian M. Delaney]: Your body is smarter than mine because if I go to bed early, the whole problem just shifts to the east. I’m in Florida, I’ll I go to bed at 9:00, I will wake up 1:00 and then I’ll try this other method of not taking a power nap so that you can have all your sleep compressed. I will fall asleep at 8:00 and I will wake up at midnight and then suddenly, I’m just back on Stockholm time living in Florida then I’m on Mumbai time. It just keeps going to the east or earlier.
[Damien Blenkinsopp]: Maybe it would be interesting if you do a CTM to see if there’s something going on; something that spikes or drops at a specific time.
[Brian M. Delaney]: That’s a good idea.
[Damien Blenkinsopp]: Then you can be like look I woke up at that time and it’s tanked like you say. Or maybe it’s not. Some people see spikes sometimes. I wonder if that’s an infection or activity during the night.
[Brian M. Delaney]: That’s a good idea.
[Damien Blenkinsopp]: Yeah.
[Brian M. Delaney]: That’s a really good idea.
(1:56:23) [Damien Blenkinsopp]: Anyway sorry. So getting back to your stack.
[Brian M. Delaney]: So I did this time-restricted, various forms of it and I’m going to keep doing that because I really do think that that can have a huge effect on health. I don’t know what my ultimate plan will be. I know I’ll do the periodic multi-day fasting or fasting periodically.
[Damien Blenkinsopp]: So just on the fast mimicking diet, you decided that you’re not sure about the research? Is that why you dropped that or is it just inconvenient?
[Brian M. Delaney]: I haven’t dropped it. To be honest, I haven’t decided yet. There’s going to be a huge conference in early November that Valter Longo is putting on at USC. November 9th and 10th that I’m going to be going to [check 1:57:00]
[Damien Blenkinsopp]: What’s that called?
[Brian M. Delaney]: Something like Fasting, CR, Longevity. It can be Googled. Valter Longo, Fasting, November, USC.
[Damien Blenkinsopp]: There we go.
[Brian M. Delaney]: I’m going to go to that and probably there will be some new results to be announced at the “Poster” sessions perhaps. So I haven’t given up on it. It’s just that I’m not convinced that it’s better than any of the other restricted eating diet. I do think it’s beneficial, but is it better than daily restriction, is it better than time-restricted window per day, is it better than every other day partial fasting? I don’t know. So that’s one thing
That was my thinking too about that up until a year ago. It was really until I met Bill and got this new amazing job. Now I can wake up and read research. That’s what I started doing and then traveling and going to conferences, talking to researches. So at that point, I realized that there were some senolytics worth looking into. How one combines that with a restricted eating is very complicated. Do you want to have two ways of getting these genes to be activated too much? Is that too much? Who knows? But Rapamycin became particularly intriguing to me. I only started it three months ago.
(01:58:15) [Damien Blenkinsopp]: Is that easy to get?
[Brian M. Delaney]: Yeah, it’s pretty easy to get. We have a relation with this group called International Aging Systems (IAS). They have a booth here and I think they’re based in London. So one can get Rapamycin of high quality source made in the EU for a reasonable price. The FDA here in the U.S or the DA, whichever it is, permits I think a three month personal supply. It’s a prescription drug.
[Damien Blenkinsopp]: So as a consumer, you can order it?
[Brian M. Delaney]: Yes and you can do it from any country. It’s just that the border controls might be tougher in some countries, but in the United States, it’s pretty open. Otherwise you can get a prescription. It’s not cheap, but it’s not like exaggerate that’s very expensive. So I’m taking now 7.5 milligrams once a week which is much higher or somewhat higher than what anyone else is taking. Typically, people take between 3.0 and 6.0.
[Damien Blenkinsopp]: Ok. Why are you taking more?
[Brian M. Delaney]: I decided it’s part of my job. I want to push the envelope a little bit. Not because it’s going to be scientific, but mostly when it comes to the side effects so that I can then report to people what I felt during that.
[Damien Blenkinsopp]: Have you noticed something?
[Brian M. Delaney]: Nothing negative.
[Damien Blenkinsopp]: How long have you been taking it?
[Brian M. Delaney]: I started at 4.0 milligrams per week about three months ago. Then I went up to 5.0 then 6.25 because I was scoring the tablets.
[Damien Blenkinsopp]: Are you taking labs or do you have a tracking routine? Or did you just take labs?
[Brian M. Delaney]: I am terribly embarrassed to say I’m sloppy on that front and it’s partly laziness, I have to confess. But mostly it’s that as part of my work, I really have to try a lot of these things and it would be so hard to isolate the relevant intermittent variable when I’m trying so many things all the time. It doesn’t add a lot of value to get labs done and to draw conclusion about any one treatment.
It’s not useless and I have done some labs and I will report on our blog at society for rescuetheelders.org. I’m going to report some results that I think I can attribute to one treatment and not some of the other ones I did a little bit earlier, but it’s complicated scientifically so I really do have to.
If I go to a conference and there’s an exhibit booth with someone I’m offering something, I feel like I have to try it. It is my job. Or if I’m traveling around the world and I meet some mad scientist who has got exosomes and I think they’re safe and he’s just, “Hey, you want some?” I’ll try it. It’s what it seems. That seems like part of what I need to do.
[Damien Blenkinsopp]: For sure, you’re training you. Every now and again, you’re using labs just to see what’s going on?
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: Just in case you sabotage your whole anti-aging plan?
[Brian M. Delaney]: Exactly. That I’m certainly doing. Another shift that I made in my diet aside from energy intake level was the foods that I eat. I want to radically high fat, low carb diet like almost 80% calories from fat; mostly nuts.
[Damien Blenkinsopp]: Macadamia?
[Brian M. Delaney]: No. I used to. I’m convinced that saturated fats of any chain length are probably not so great. Macadamia nuts have more saturated. It’s not like steak or lard. If I’m at a party, I’ll grab quite a few, They’re delicious, but no. I try to stick with walnuts, almonds, pistachios are really good. What else? Pecans; those are not so great, but I love them. A little bit of olive oil which also has a lot of saturated fat compared to some of these other nuts. That shift has led to higher LDL.
Just to speak, I generally track my biomarkers. The one thing I’m worried about that doesn’t look good is I haven’t done my NMR; this wave measure particle size of the LDLs. I haven’t done that in a while. The last time I did it, it looked good, not great, but good. I have to do it again so I’m eating even more nuts now. I wake up, I eat nuts and broccoli or kale. That’s pretty much it.
So now back to Rapamycin. I started increasing the amount I was taking and really I’ve had no side effects. I had cankers; a canker sore once which people get. Only once.
[Damien Blenkinsopp]: So one of the side effects of Rapamycin is immunosuppression so that’s one of the concerns. That’s why you’re not taking it every day because you’re going to get some immunosuppression, but you’re hoping that that’s just a momentary downside and possible canker sores.
[Brian M Delaney]: Yeah, almost everyone reports that. I would not recommend 7.5. That’s quite high. But on the other hand to be honest, if you scale up from the rodent studies that showed the maximum lifespan benefit, the equivalent would be something between 10.0 to 12.0 milligrams for a human once a week. That’s part of why I bumped it up to 7.5. I may even go higher. We’ll have to see.
It has a really long half-life. Usually some people say I think between 62 and 67 hours. So one can do 7.5 and maybe do it every eight days instead of every seven just to give some period for letting it to taper out.
It has probably, as you said, the immune risk, but also there seems to be a risk of glucoregulatory dysfunction. It’s not clear.
(2:04:05) [Damien Blenkinsopp]: In terms of it’s more variable?
[Brian M. Delaney: No. Actually glucose goes up.
[Damien Blenkinsopp]: That’s the general level trend?
[Brian M. Delaney]: Yeah it goes up in some studies, not all. But then there’s this other weird phenomenon where it seems to disappear after a while after a few months. That’s why there’s this problem. We’re pretty sure we have to pulse the dose, but is the pulsing done once a week or once a day? Or take it once a day for a few months, let the side effects taper off which they do, according to some studies and then stop it.
[Damien Blenkinsopp]: Then restart?
[Brian M. Delaney]: Then stop it. We don’t know.
[Damien Blenkinsopp]: So even pulsing once a week, you’d still get that rise? Is that a chronic dose?
[Brian M. Delaney]: We don’t know yet. We don’t know yet. Actually, here I do have some data. This is something that I can say it’s the Rapamycin. It’s a good [check 2:04:54]. Now I’m eating very early in the morning and a smaller amount fairly late in the evening. So my big gap is actually between breakfast. So I take my fasting glucose at 8:00 p.m. and it is typical these days, so not on extreme CR.
It’s in the mid-seventies. On Rapamycin, it has been typically 70 or 71 so it has not gone up, it has gone down. The margin of error is pretty large, but it certainly hasn’t skyrocketed upwards which is what some of the mice research indicated it would.
So I’m not worried about that effect, but I haven’t had my lipids measured recently, but I am going to do that soon. I’ll do a full NMR and see what it looks like. That’s part of my stack. We could go one for hours here.
[Damien Blenkinsopp]: So we have time-restricted eating?
[Brian M. Delaney]: Of various forms, yeah.
[Damien Blenkinsopp]: Of various forms. We have ketogenic diet?
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: We have Rapramycin.
[Brian M. Delaney]: 7.5 milligrams.
[Damien Blenkinsopp]: 7.5 milligrams.
[Brian M. Delaney]: Probably will go higher.
[Damien Blenkinsopp]: What else?
[Brian M. Delaney]: The next would be nicotinamide riboside; oral nicotinamide riboside.
[Damien Blenkinsopp]: Is that Niagen?
[Brian M. Delaney]: That’s one of the brands, yeah. This is to raise NAD levels in the blood and more importantly in the cells.
[Damien Blenkinsopp]: Have you done any testing of that? Because I saw people are doing more tests now. I haven’t seen any results
[Brian M. Delaney]: Of NAD levels?
[Damien Blenkinsopp]: Yeah.
[Brian M. Delaney]: No I haven’t yet. This is a complicated topic. Do blood levels matter so much or is it the levels in the cell?
[Damien Blenkinsopp]: Red blood cells. Yeah, exactly. Well, yeah.
[Brian M. Delaney]: So I’m not really sure, but we do know that nicotinamide riboside will raise blood levels. It will double blood levels. I’m not sure how much we know the extent to which it will raise levels in the cells, but it certainly does raise levels. This is something in the cells which is where it matters.
Because Rapamycin is a partial CR mimetic, it’s probably going to increase my own production of NAD to some degree. So I have this complicated weekly cycle of when I first take the Rapamycin, I’ll only take 250 milligrams of nicotinamide riboside those first two days per day and then I’ll go up to 500 and then towards day six or seven, I’m taking 750. Then when I’m doing a fasting mimicking diet, and by the way I may skip a week of Rapamycin, I may adapt that pattern. So once a week, four to six weeks, do a five day partially near fast and then don’t take Rapamycin because that would be too much; little bit too much going on at one time.
[Damien Blenkinsopp]: You’re doing the fast anyway.
[Brian M Delaney]: Then I wouldn’t take any nicotinamide riboside for a few days. So time-restricted eating, ketogenic diet, high-fat, low-carb, Rapamycin, nicotinamide riboside. Maybe I’ll do the occasional NAD patch or infusion. I don’t know. I’m not really sure about that. But then finally, of the big things exercise of course which you know of exercise.
(02:08:00) [Damien Blenkinsopp]: What kind of exercises do you do?
[Brian M. Delaney]: Strength training. I have so many old baseball injuries, there’s not a lot I can do. I do pushups. I have a chin-up bar. I certainly don’t have time to go to the gym so I’ve got my backpack with different sized rocks I put in it and do overhead pull-ups. I do everything at home so I just can’t think [unclear 2:08:21]. Then I run and walk briskly.
I always exercise after meals to knock down those blood glucose and lipids. People don’t realize postprandial lipids can be a problem too. So always. If I’m at a restaurant with a billionaire that I’m trying to get financing for a project, maybe I won’t exercise and I will take Metformin.
(02:08:46) [Damien Blenkinsopp]: Are you taking Metformin as well?
[Brian M. Delaney]: Only if I cannot exercise after eating and I’ve had more than a tiny amount of carbs.
[Damien Blenkinsopp]: Ok. Interesting.
[Brian M. Delaney]: Five hundred milligrams.
[Damien Blenkinsopp]: Is that based on any study or anything?
[Brian M. Delaney]: Well it’s based on what we know for Metformin and Type 2 diabetics. It will actually knock down those [unclear 2:09:04].
[Damien Blenkinsopp]: Some people are just taking it chronically.
[Brian M. Delaney]: Lots of people.
[Damien Blenkinsopp]: Yeah.
[Brian M. Delaney]: Lots of people.
[Damien Blenkinsopp]: More and more.
[Brian M. Delaney]: Tons of people. I’m actually an outlier here where I’m actually not convinced that for healthy, trim people who eat well and exercise that it really is worth it. I know some clinicians who have tried it. This is actually worth noting. Two clinicians who are anti-aging doctors, really smart people who have tried Metformin in elderly and the elderly say, “I feel like crap” because it lowers their energy levels. That’s part of how it works.
So now the next thing I have to talk about is the senolytics. A lot of people think that we shouldn’t try any of them; that we need more human research. Not that I can’t understand. It’s not strange for a physician to say let’s wait until phase one, phase two, phase three trials are out. That’s why we have not recommended that anyone tries these. I can just say that I personally want to and have tried them. I want to try them and have tried them and would like my mother to try them.
The combination of the Dasatinib and Quercetin, if one is going to try it, the conservative thing would be do it once every few years. You just take the dose, knock out a whole bunch of certain classes of senescent cells. It doesn’t target all. Each different type of senolytic agent has a different target. So this is mostly preemptive sites that it knocks out and a few other types of cells, but mostly senescent cells. Do it once every few years and then they go back.
I’m doing a much more aggressive approach where I’m taking it every four or five months. I should stick to a strict schedule, but I just get too busy. I’m traveling. There can be side effects for this during the 12 to 24 hours after you take it.
[Damien Blenkinsopp]: What’s the name of those?
[Brian M. Delaney]: This is Dasatinib which is cancer drug.
(02:11:10) [Damien Blenkinsopp]: How do you get your hands on that?
[Brian M. Delaney]: Metaphorically speaking, sometimes it’s a researcher pal in some way.
[Damien Blenkinsopp]: Some connections?
[Brian M. Delaney]: Yeah. You can get it various ways, but the proper way to get it which I’m now going to use is me to go to a doctor. I mean researchable. I’m not talking about anything illegal. You become part of the study and there are lots of studies now going on; not lots, but a few. But the standard way that I would recommend would be go to your doctor, your healthcare practitioner, say you want to do this, show the doctor the studies and get a prescription and then pay for it.
The ways to get this through various overseas sources where it’s a little bit less expensive, but still high-quality, usually through India. The amount you take is per round which you can divide in half and then take one week and then the next, but per round would be 5.0 milligrams per kilogram of body weight.
[Damien Blenkinsopp]: Ok.
[Brian M. Delaney]: So that would be 750 milligram tablets. It’s actually hard to get a bottle of 750 milligram tablets. So I usually have to buy too much and then give some to a friend or go halves on it.
Quercetin is a natural substance it is a supplement. You take ten times as much of that so it would be 50 milligrams per kilogram of body weight. So what we now think is that it’s better, and Bill Faloon will be describing this, we meet lots of people. It’s better to divide that in half per what you call round you do once every three years or once every four or five months.
[Damien Blenkinsopp]: So it’s not very frequent.
[Brian M. Delaney]: No, no.
(02:13:04) [Damien Blenkinsopp]: So what about all these vegans eating [check 2:13:05]?
[Brian M. Delaney]: They’re not eating as much.
[Damien Blenkinsopp]: Not as much as they need.
[Brian M. Delaney]: No, not even close to what you get from this protocol. So you take that and you might get muscle cramps. The one serious risk which is theoretical, never heard it happening to anybody, is anaphylactic shock
[Damien Blenkinsopp]: Ok.
[Brian M. Delaney]: The smart thing to do is to go to your healthcare practitioner and talk about it and if it’s ok, he or she will say no don’t do this because it’s crazy.
[Damien Blenkinsopp]: You would think a lot of cells are getting killed off.
[Brian M. Delaney]: So the theoretical risk of the dangerous side effect is anaphylactic shock. The more long-term theoretical potential downside is the off-target effects because the mechanism is such that it could kill some healthy stem cells.
(2:13:59) [Damien Blenkinsopp]: I’ve got a silly question. If we’re killing off all those senescent cells, let’s say they’re doing something, but they’re just not doing it very well and as stem cells are declining, are we able to rectify that? Do we end up with enough cells to do the job?
[Brian M. Delaney]: What the senescent cells are doing, mostly they’re doing really bad stuff, but there are some positive roles with tissue remodeling.
[Damien Blenkinsopp]: They’re trying to do their job.
[Brian M. Delaney]: No, it’s not that they’re trying to do their job and they’re kind of doing it, they’re not doing it at all except possibly the tissue remodeling and sending out these extracellular matrix proteins that some of them are dangerous, but some of them are actually useful. They’re useful in tissue remodeling. So I don’t think that’s the problem.
The theory behind why this helps with osteoarthritis in particular; that’s another thing seen in rodent studies and I think I can say there’s some human data. Yeah, I will say that we have some human data that it really helps in osteoarthritis. The way that it works is it actually frees up existing stem cells to do their job. But in theory, we really don’t know. I see the results in the humans and I see the results in the rodents.
I had a kidney stone a year ago; more than a year ago. It was diagnosed with a CT scan. I discovered I have calcification. Not much, very little, but that really shocked me.
[Damien Blenkinsopp]: So you had the calcium scroll?
[Brian M. Delaney]: No, I just discovered. They saw that in the CT scan. The guy asked me, “Do you smoke?” No. You have some calcification in here, not much. It was a shock. That’s one of the reasons why I was motivated to go on an aggressive Dasatinib and Quercetin treatment protocol because it seems I have some calcification. So theoretically I could be doing some harm.
So reason someone who is really young, I would say, “My God! Don’t be absurd to do this.” Having anyone under 40, certainly it seems foolish.
[Damien Blenkinsopp]: Something to do later.
[Brian M. Delaney]: Yeah, and that’s partly because the potential off-target effects. We really don’t know. We have to do those studies. Bu there’s another reason. It’s not entirely crazy to think that some point soon, we can turn some of these senescent cells back into healthy cells.
[Damien Blenkinsopp]: Turn them back; fix them.
[Brian M. Delaney]: Yeah. Exactly. People are now talking about senotherapy is this new term. Basically you deal with the senescent cells in various ways, not simply with senolytics which destroys them, but there is a new term that I think now rightfully could be applied to Rapamycin, called a senomorphic. It changes the senescent cell. It doesn’t make it perfectly healthy, but there is evidence that Rapamycin will lower the amount of these injurious paraben factors that the senescent cells are letting out. So they’re morphing; they’re changing.
[Damien Blenkinsopp]: They’re less antagonistic.
[Brian M. Delaney]: Yeah, basically. So Rapamycin actually has that effect. Presumably CR does as well.
[Damien Blenkinsopp]: Is that damaging them in some way maybe?
[Brian M. Delaney]: Damaging the senescent cells?
[Damien Blenkinsopp]: Yeah.
[Brian M. Delaney]: Probably not. Probably not. We don’t know, but I would imagine it’s more of an epigenetic change in the senescent cell itself that’s actually changing.
[Damien Blenkinsopp]: [Unclear 2:17:27]
[Brian M. Delaney]: Yeah. Have these injurious [check 2:17:31].
(2:23:00) [Damien Blenkinsopp]: Great.
[Brian M. Delaney]: That’s it.
[Damien Blenkinsopp]: That’s full stacks for you?
[Brian M. Delaney]: For now. We didn’t mention the biologics. We did; we opened with the biologics. The next categories would be the biologics. The newer “Living Medicine” as some people are calling it which doesn’t apply to plasma, but does to cells. If I had the money, I would get, people are now saying MSCs which used to stand for Mesenchymal Stem Cells, but now they’re saying let’s call them Medicinal Signaling Cells.
[Damien Blenkinsopp]: I heard that last night.
[Brian M. Delaney]: Because it’s not clear that they’re stem cells. [Check 2:17:59] mentioned that Mesenchymal Stromal Cells, but anyway MSCs from birth-associated tissue. I have not done that yet, but if I could afford it, that would be my next step.
[Damien Blenkinsopp]: It looks interesting.
[Brian M. Delaney]: And young plasma as young as possible from umbilical cords. Cord blood would be great. What Howard Chipman’s offering is also very good as I mentioned earlier. I felt like Superman for a day and a half; tragically short, but it was great. So that would be the thing that I would really want to put a lot of energy into for my own treatment next if I go forward.
(2:18:32) [Damien Blenkinsopp]: Great. Wow. So are you doing any consistent tracking? Is there a lab panel you do once a year or once every six months?
[Brian M. Delaney]: What I’m going to start doing is…
[Damien Blenkinsopp]: Or am I encouraging you?
[Brian M. Delaney]: You are and lots and lots of people think I’m being an idiot or lazy or both by not getting more blood work done. I track simple things like pulse and blood pressure and body temperature at home. Body weight obviously; I’m doing that for years. Bill Faloon and I and our team came up with this age management profile that you get at lifeextension.com. I think there’s a discount. Let me see. I will make this available.
[Damien Blenkinsopp]: It’s a set of panels?
[Brian M. Delaney]: A huge set of things. I am going to do that every six months and it has got a whole bunch of relevant markers. It has got a lot of inflammatory markers. The other thing I’m doing is DNA methylation testing; Zymo Research Program.
(2:19:43) [Damien Blenkinsopp]: DNA methylation?
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: So epigenetics.
[Brian M. Delaney]: That may be new to some of our viewers.
[Damien Blenkinsopp]: That’s really new. I’ve been talking to a couple companies doing that. There’s one in the U.K. It looked interesting, but I was like it’s the early stage. I got into the whole microbiome area. I’ve done so many tests from all the companies, nothing actual. The test results were varying between companies. I was like you know what this is too early stage so I don’t know if this is actually usable at all.
So now I take my time, talk to a lot of people, try to get into it. It’s easier if I take two labs and I put them together, am I going to get some similar results or what am I going to get here?
[Brian M. Delaney]: With epigenetic testing, you will get varying results sometimes.
[Damien Blenkinsopp]: They’re changing off.
[Brian M. Delaney]: Exactly. Steven Horvath at UCLA is the one who came up with this idea, I think. He’s the one that came up with it. He selected, they’re called CpG sites. They’re a particular area between a C and a G in a DNA chainwork. You’ve got potentially a methylation, you’ve got a group covering over the DNA so that it can’t be expressed. I think he based on actuarial data and or data from the [unclear 2:21:08] study, but they had tons of data and it is very accurate way of predicting someone’s chronological age.
[Damien Blenkinsopp]: Chronological or biological?
[Brian M. Delaney]: Chronological.
[Damien Blenkinsopp]: So no matter what you’ve done during your life.
[Brian M. Delaney]: Oddly enough.
[Damien Blenkinsopp]: It will still say you’re 50.
[Brian M. Delaney]: Well no, it will vary a little bit, but the goal is chronological age. Now, he has now come up with something called a phenoage. It’s the new selection of CpG sites. That’s going to measure biological age. That would be more useful. Zymo, this company that had a license with Horvath, they have a blood test, a urine test and I think a saliva test. For each one, I think it’s a slightly different selection of CpG sites.
[Damien Blenkinsopp]: So you have to do all of them?
[Brian M. Delaney]: One could do all of them. I am doing all of them. I am doing all of them.
[Damien Blenkinsopp]: That would give you a more complete picture? You plug this into an algorithm?
[Brian M. Delaney]: No, they don’t do that. Well they could, maybe they will, but this is off the books thing that we’re doing. They’re helping me and I’m helping them, I hope by giving them more data.
[Damien Blenkinsopp]: Are they early stage in this business?
[Brian M. Delaney]: I would say it would be somewhat early stage.
[Damien Blenkinsopp]: But it’s based on Horvath’s work?
[Brian M. Delaney]: It’s based on Horvath’s work and their own. They’re now doing their own research. So I think they’re going to eventually move towards their own selection of CpG sites that they think will be the most useful. They may have several different tests. One that may be useful for measuring chronological age license to insurance companies. One that would be a measure of phenotypical age; your actual biological age of people like you and me and a lot of people watching this.
That I’m doing. I believe in the idea. I agree with you that it’s somewhat early, but only somewhat. I had my last results when I was 54. Came out as 50 which is ok as a guy who started CR too late, kind of went off CR for a while because I enjoyed the testosterone. Ok that seems like four years. I’ve done strict CR earlier even though it’s supposed to be chronological and not biological, it does change with increased production and aging. So maybe it would’ve been 47 instead of 50. I don’t know. Yeah, but it was nice that it was younger than.
[Damien Blenkinsopp]: Validation that it wasn’t all a waste of time.
[Brian M. Delaney]: Exactly.
[Damien Blenkinsopp]: That’s what we’re trying to get out it. Some really good biomarkers to tell us that the stuff’s working.
[Brian M. Delaney]: Yeah.
[Damien Blenkinsopp]: Man, this has been a great chat.
[Brian M. Delaney]: Yeah it really has been. Thank you.
[Damien Blenkinsopp]: Thank you very much. We’ve covered so much stuff and it has been great to hear your personal experience and what you’re up to. Your personal journey really, you’re constantly modifying stuff and looking into new stuff. So where can people get in touch with you or reach out to you or learn more about what you do?
[Brian M. Delaney]: We have a new website with a tragically long URL, but it’s not too hard to remember. It’s scoietyforagereversal.org and there’s a blog there. If you click on that, I haven’t started it yet. I’m going to start it tonight.
[Damien Blenkinsopp]: So tomorrow there’s going to be an awesome post.
[Brian M. Delaney]: Yeah, I hope. I hope.
[Damien Blenkinsopp]: Depending on how long it goes on this evening.
[Brian M. Delaney]: Exactly. Excatly. It may be somewhat a little bit drunken depending on the party tonight. Actually alcohol is not something one should partake in too much if one wants to live a long life. That’s where I’m going to be updating people on what I’ve discovered and side effects of my own experimentation [check 2:24:31]
[Damien Blenkinsopp]: Great.
[Brain M. Delaney]:Thanks a lot.
[Damien Blenkinsopp]: Thank you so much for your time.
[Brian M. Delaney]: You bet. Thank you.
(2:24:44)[Damien Blenkinsopp]: Third time lucky. Hey guys! We’ve been messing around with the equipment here, but we’re now ready to chat. So right now, we’re at RAADfest. This is [check 2:24:53]. We’ve got today and tomorrow left and we’re going to be doing some more interviews. Right now we have Quantified Bob. You know Quantifed Bob if you’re a superfan of Quantified Body because he was in episode 22 talking about intermittent fasting and blood glucose dysregulation and his experiments in tracking around that.
So you met Bob and we had a great conversation before. So if you haven’t listened to that, you might want to go and listen to that before you relisten to this or rewatch this because we’ll just cover new ground basically. We’re not going to go over the old stuff. So Bob, how are you doing?
[Bob Troia]: I am doing great. This is the third day of this RAADfest event. So we’ve had so many conversations just over the last three days because to see some of the talks about some of these advancements and some of the therapeutic work that’s being done around stem cells. I think when I was on your podcast, it was three years ago.
[Damien Blenkinsopp]: It was a long time, yeah.
[Bob Troia]: We were talking about it on a very macro high level certain dietary things and interventions, getting into maybe some data around glucose tracking, but now we’re getting down to the cellular level and subcellular level and seeing how rapidly these advancements are happening. It’s really cool. Just in those past few years, I think we’ve gained so much additional knowledge and insights. I looked back on even when we spoke and I was like, “Wow” some of it is actually cool. It’s relevant.
[Damien Blenkinsopp]: It’s still relevant.
[Bob Troia]: We talked about [check 2:26:17] testing, but then there was a whole new wave of science and things that are coming out.
[Damien Blenkinsopp]: Yeah. What have you thought about the event so far? Are you enjoying it? Are there great points? Would you recommend people come here?
[Bob Troia]: Yeah! I haven’t been to this event before. That was my main curiosity about it. I wanted to come last year and I didn’t so I came this year. I didn’t know what to expect. I’m a long time subscriber of the Life Extension. You get the magazine and some of their supplements. It’s a really cool, interesting crowd.
There’s a real sense of community around this. We talked about longevity and even this conference is called Revolution Against Aging and Dying and I’m just like ok. I like to make it a little positive. Instead of saying revolution against, I would make it something for living longer and better and more productive lives.
But the talks have been great. I like conferences where the presentations get into some science, not being a sales pitch. So we’re getting to see some really cool talks. I’m actually learning some more. Often when I go and I see talks, it’s like I’ve read about this, I’ve already read that and read that. Yeah I’m coming away from these talks with notes and mental notes and things I can go follow up on because it really piques my interest.
(02:27:34) [Damien Blenkinsopp]: Yeah. So I’ve also found that the people we met here, I’ve met so many cool people here and talking with people. We hung out with people last night from some of the startups that are being funded in order to bring some of these anti-aging therapies eventually to market. So there are really interesting people here doing interesting stuff so I think that’s one of the great things. This is my first Life Extension conference. What about you?
[Bob Troia]: Yeah, same. The other thing I was going to say was that what we’re seeing is this general idea, in the general space of this whole wellness and longevity, the overlap because I’m running into friends and people. I was like, “What are you doing here?” From whether it’s a quantified self conference or a biohacking conference or a biohacking conference, all these worlds are just overlapping now. It’s really interesting.
[Damien Blenkinsopp]: You’re meeting the same people.
[Bob Troia]: Yeah. Well new people, but also you’re seeing everyone’s interests are cross-pollinating. It’s all becoming around this whole concept of overall self-optimization and figuring out all the different ways to make ourselves as best as we can be.
(02:28:39) [Damien Blenkinsopp]: Excellent. All right, let’s talk about what you’ve been up to since we last spoke. So it has been three years, what’s the most interesting tool or tactic you’ve tested or you’ve used consistently because you actually see it’s making a difference?
[Bob Troia]: Sure. If you want to talk about just insights, I feel I’ve gone from again that macro level of tweaking my diet, trying to heal my gut and those types of tactics. We talked about concepts like intermittent fasting and now you’re seeing proliferation of things like types of fasting protocols and fasting mimicking diets. So if we’ve both done a lot of experimentation around that which was really cool and digging even a little deeper.
Ultimately everything we’re doing boils down to, for me at least, I’m seeing it as mitochondrial efficiency. So I look at tools and tactics and be like how is that helping or injuring that. So whether I’m using modality that reduces oxidative stress in my body or [check 2:29:42] in my diet, it’s all how about I can make it as efficient as possible. So it’s going back to as you peel back every layer of the onion, you’re going, “Ok, what am I really honing in on there?” Yeah so for me, that’s a really big part of it.
Some of the tools, we talked about wearables and getting data off all that and we’ve seen the big ship to that whole landscape.
[Damien Blenkinsopp]: A lot of companies are gone.
[Bob Troia]: They’re gone. Or the ones that were really open about letting you access data and have open access to it, they’re siloing themselves off because they’re trying to monetize it on their own which has been frustrating. But three years ago, everybody was doing 23 and me testing for genetics, but now whole genome sequencing is affordable.
(02:30:24) [Damien Blenkinsopp]: So we’ve seen a few companies talking about this; the whole genome sequencing thing. Liz Parrish’s BioViva, I’m doing that, Health Nucleus is doing it, but there are other companies as well. I told someone yesterday. He was saying there’s a huge movement in China for this whole genome sequencing so it’s available now. It’s actually the whole thing rather than the 23 and me is just a small part of it. So we’re getting to that step where we actually have better data.
[Bob Troia]: Yeah and it’s one of those things where five years ago, that whole genome sequencing was a million dollars and now it’s down to under a thousand dollars and a year from now it will be what you’re paying for 23 tests a few years ago. It’s pretty amazing. I think the fact that other people are doing this, that’s going to help bring this cost down because they’re all competing in a way now.
(02:31:09) [Damien Blenkinsopp]: I guess the other thing I liked about this here is this community. You see these companies, they’re competing against each other. Like stem cell companies, they’re in the same area so they’re competitors, but what you see here is everyone has a common objective which is to defeat aging and to defeat the damage of aging.
They’re working together a lot of the time in networks and in partnerships even though they’re actually competitors. So it’s really nice to see that because they’re so much focused on the objective, they’re like I don’t care who makes it. It’s like Elon Musk. He’s like I just want to let electronic cars be in the world so I’m going to open source the info.
[Bob Troia]: Even seeing the Life Extension Foundation, they fund a lot of research and they’re funding companies that are essentially viewed as competitors, but they’re going to get some innovation over here and we’re going to get some innovation over here and eventually it all will start coming together. Yeah it’s pretty cool to see. I’m also seeing companies are getting funded.
[Damien Blenkinsopp]: Yeah. It’s a lot of funding.
[Bob Troia]: Institutional money, big money and I was like wow.
(02:32:14) [Damien Blenkinsopp]: So we saw the SENS Research Foundation. Yesterday they had Y Combinator, part of Y Combinator had invested in one of their companies targeting aging. Andreeseen Horowitz so these are huge names in the BC incubator world.
[Bob Troia]: Yeah. I think they all see where this stuff is going and they’re putting their bets down now on some of these players. I come at it from I have technology background, I’m an entrepreneur as a UN so it’s interesting to watch how it all plays out because some of these are areas that maybe were more risk-averse to a few years ago. Now they’re seeing our studies and they’re seeing some glimmers of hope there in terms of wow they’re really on to something so there’s money going in there. For me, it’s about ok I want this company to be be successful and funded so they can make these things available to me at an affordable cost.
Yeah that’s pretty exciting, but back to your original questions about what I’ve been up to the last few years, I’ve gone through a lot.
(02:33:12) [Damien Blenkinsopp]: So what are you doing in a typical week now? You get up in the morning, what does a typical week look like now in terms of the tools and tactics and the tracking?
[Bob Troia]: So I look at it from the standpoint of no matter if one person’s data is more optimized than the other, it really comes down to we all have 24 hours in the day and how am I going to make the most of that time. I’ve been fortunate in that I’ve done a lot of this light work in terms of collecting some of my data, looking at data. I’m not doing it all the time.
There are moments where I might do continuous glucose monitoring for a period of two weeks, but I’m not always wearing that sensor because I got my insights for those two weeks. I maximize the time I’m wearing it to get my insights. Maybe six months from now or a year, I’ll use it again. So that’s not a burden on me.
I try to possibly collect as much data as possible. So even if I might not be using it today, but if I want to go back and look at what I was doing six months ago what actually happened back then, the data is there. It would require no effort on my part. I spent a lot of time in the few years back setting up some systems and tools and now it’s very much like a set it and forget it kind of thing where it can be on autopilot to some degree.
What I’m seeing now even on the consumer side is the frustrations where they’re getting access to their data and tools, but the insights they’re getting are not. You might say how your sleep is terrible, but hey your sleep is bad. I know my sleep is bad so what should I do and they’re not really being given that next step above what tactics and tools and what they could be implementing. There could be a whole slew of issues related to why their sleep is poor and really digging into that.
Things like training and recovery. I’ve been really big on exploring some of these devices and tools and modalities that help us. Instead of going to the gym six days a week for three hours a day, literally just 30 minutes here and 30 minutes there and be on with your day and you’re going to get just as much result out of this. It’s not about who can work out the longest and who can push the most weight. There are more efficient ways to do it.
In terms of supplementation and experimenting with different things whether it’s nootropics or just making sure I’m getting proper nutrient balance in my diet, I definitely cycled on and off things. Right now, I’m three months into taking this nicotinamide riboside product that’s basically a precursor to NAD. The body should be able to convert this into that. I’ll be getting some blood drawn shortly to see has it shifted. Has it increased my levels? Compared to what someone my biological age would have.
In terms of is it something I’ll be taking long term? I may not need it. It may be the result is actually your body doesn’t need that additional supplementation. Maybe if you were a different state or condition or older then it would be ok. Maybe ten years from now, you should consider starting to take it. But I’m seeing just other observations with it. It has a slight shift to my circadian rhythm. I was waking up about 30 minutes earlier a day, but not exhausted. It just seems it made me want to wake up earlier. Recovery from workouts and training definitely were a noticeable effect of it. That’s just one experiment.
I’ve been doing a lot of stuff around cognitive testing and understanding how to find tools that can help measure and assess whether you’ve got acute trauma or past trauma in your brain or fatigue, et cetera and then what can you do or take, what helps or hinders that. Because I had actually thought, I assumed from playing sports for years, getting hit in the head repeatedly, I’d have some issues. But it turns out some of the tactics I guess I have been doing over the past few years have kept my brain state at a good level.
When I did the assessments, it was actually I’m not saying disappointed, but everything was really good. There were like you don’t really need to make any changes or just keep doing what you’re doing.
So we’re seeing these cool assessment tools and devices are coming out of these labs and maybe they’re used by professional sports teams or the military and they’re being made accessible to basically anybody. Part of what I’ve done is I have all these different types of training and recovery tools.
About six months ago, myself and another person set up a facility in New York City because I was realizing friends were coming over to use a lot of the things I had. So instead of me just eating the cost of one of these devices, I was letting people get some benefit out of it so I said why not just put it into a space and let people come and share it without having to come to my home.
So it has been fun. It’s almost a little part-gym, part-lab, part-playground and so that for me is really exciting. From a business standpoint, really I just use it more as a place where I’m collecting data and I can do some really cool experiments around training and recovery and figure out how I can use these tools to effect based on biomarkers.
(02:38:12) [Damien Blenkinsopp]: Yeah. Is there anything consistently you collect and do daily or at least weekly, over time?
[Bob Troia]: Yeah. Daily, my routine would be as soon as I wake up in the morning before I even get out of bed, I do a hurry variability check. So about a two-minute check.
[Damien Blenkinsopp]: Are you correlating it with the aura?
[Bob Troia]: Yes, they do correlate. This is the new aura ring. So overnight while you’re sleeping, it’s taking heart rate variability readings throughout the evening and then it gives you an average number.
[Damien Blenkinsopp]: For the night?
[Bob Troia]: Yeah and it will vary.
[Damien Blenkinsopp]: It gives you the peak as well?
[Bob Troia]: Yes. So you might go from really low sympathetic state to a parasympathetic, but it’s just going to average it all out. So you may have had a really poor night’s sleep, but there might have been a part where you had really high HRD, good HRD so it hides the fact that you had a poor. Otherwise when you wake up in the morning, if you had trained really hard the day before or you’re jet lagged, you might see it’s suppressed today. They’re different, but they’re both important. They both give you a different insight into your physiological state.
If you go from that, you’re obviously sleep tracking. You can then start looking at the effects of that and I wear that with other types of data. If I’m home, I kind of understand my environment, my bedroom so air quality, temperature, humidity, light, sound and then things like body composition. The scales are not the super most exact body scales.
You can’t miss 4% body fat in a day, but if you just blur your eyes and stand back and look at the trend over six months, you will see the trend and you can point out where, “Yeah I changed my workout. I was lifting a lot more heavy weights during that month” and you can see the changes there.
Glucose tracking, I’ll do spot checks with a finger stick. You do a fasting reading so before you have any food or drink. Ketones as well, you can do that. Then I’ll play around throughout the week maybe if I actually want to see glycemic response to different foods. I can do spot checks with the finger stick. From the ketone measurement, for a while I was doing testing with the strips. I think if someone is more keto-adapted, actually it might make you look like you’re not in ketosis; you’re not doing it.
[Damien Blenkinsopp]: Blood ketones?
[Bob Troia]: Urine with the strips.
[Damien Blenkinsopp]: [Unclear 2:40:41] urine.
[Bob Troia]: Yeah because your body won’t be excreting it. Your body is keto-adaptive. You know this.
[Damien Blenkinsopp]: Some guys won’t know it.
[Bob Troia]: Yeah. Sure. Well basically with ketones, there are three ways to measure them. You can do blood, breath or these strips if you use urine. There are different proxies to basically the levels of ketones in your body, but blood is the best way to really measure them. I think you probably use the same meter. There’s a meter that you can use for the glucose measurements and the ketone measurements. Breath is an interesting one because it’s using acetone from your breath, but I can’t get it to get consistent readings.
[Damien Blenkinsopp]: I’ve got PhDs looking into this at the moment and it’s really tricky. The devices we have for tracking breath ketones at the moment, very, very tricky to use so we’re re-evaluating whether we should continue or not, but yeah we’ll find out. We keep on digging to try and find out. Because it appears that the meter actually measures other things and that can interfere. Basically you’re getting a combined reading of acetone and something else.
[Bob Troia]: Yes.
[Damien Blenkinsopp]: Depending on what you’ve eaten or your gut bacteria, potentially you get a signal and you think you’re in ketosis.
[Bob Troia]: Even if the force of your breath is not super consistent.
[Damien Blenkinsopp]: It’s really hard to control, yeah. The blood ketones actually go down over time as you get more keto-adaptive. So mine have gone down, not hugely, by about 1.0 millimolar. So I used to be nearly 4.0 sometimes in the afternoon. Now I’m being more 3.0 or even 2.5.
[Bob Troia]: Wow! That’s really good. I’m not going to say my diet is a keto-diet, but through just my normal diet and periods of intermittent fasting, I always wake up in the morning in a state of at least mild nutritional ketosis. So it’s fairly low, mild, but I can shift really easily into a higher state if I just fast for a day without taking exogenous sources of ketones.
So I don’t know if I mentioned that. I had done some experimentation with pure ketone esters. But most people, like athletes, Tour de France cyclists, are using these super big energy boosts.
[Damien Blenkinsopp]: Use the KetoneAid ones?
[Bob Troia]: There’s a product called KetoneAid that was pure beta-hydroxybutyrate. It is the worst-tasting. It is like rocket fuel so you have to chase it with a little bit or mineral water. It’s really crazy.
[Damien Blenkinsopp]: To wash your mouth out.
[Bob Troia]: Yeah. I approached it from all these athletes are doing it and reporting on benefits from athletic performance, I was like I want to see what it does to cognitive performance. So I did an experiment around just a whole battery of cognitive tests where I established, for two weeks, I just got my baselines. I got rid of any learning effects so the scores that couldn’t get any higher, I leveled out. I’m not getting any smarter, better or faster, my reflexes.
I took this product, it was a very small amount, but it was super, super powerful. Within 15 minutes, I used a blood ketone meter. They only go up to 8 milliomolars, the upper limit. I went, it had an error message. We were through it. So basically the dosage, I should have taken maybe half the amount.
[Damien Blenkinsopp]: How ddid you feel?
[Bob Troia]: It’s just a weird experience. Everything is brighter, your mind is lit up. I was nervous for a millisecond because I feel the gears shifting like an engine’s revving up, but then you’re just like whoa this is amazing. Your brain is never getting that sort of just flood. I mean it is pure beta-hydroxybutyrate getting right into your brain. You’re just like wow.
[Damien Blenkinsopp]: So what were the reults on that?
[Bob Troia]: We waited an hour before I did the tests. So I took it, went off the charts in the meter. Then we waited an hour so it got back down into the range of 6.0 to 7.0 millimolars as soon as it was therapeutic kind of zone and redid all the tests. The battery of every single one, I immediately increased in my scores over those baselines. These test everything from working memory to speed and reflex. It’s a battery of things, but all the scores across the board went up as high as 35, 40%. I was just like this is crazy. Then I go, “This can’t be.” Maybe I have adrenaline going.
The ketones didn’t last. The window of time is four hours.
[Damien Blenkinsopp]: For the ester?
[Bob Troia]: Yeah they tail off and you’re back to normal. So the next day, I was like let me go back and do them again with no esters, my scores were my baseline scores. So it was a temporary bump.
[Damien Blenkinsopp]: Ok. That’s interesting because one of my friends in the U.K., he got the DeltaG one which is the one that humans use. He did a weeklong test taking it every day and it was similar. The first day had all the anecdotal I feel different and the other days, it didn’t seem to make as much of an impact. He seemed tolerant.
[Bob Troia]: Oh really?
[Damien Blenkinsopp]: Yeah.
[Bob Troia]: I wasn’t taking any of the esters. I took them once. So it was almost I was going from zero.
[Damien Blenkinsopp]: I mistook you. I thought you were taking it the next day as well.
[Bob Troia]: No. I never took the esters.
[Damien Blenkinsopp]: So it’s not building your brain better. It’s temporary.
[Bob Troia]: It’s a performance enhancer I would call it. It’s very expensive so I think for athletes who they’re going to use it more often for performance, but if I’m going to go on Jeopardy, maybe I’ll pop it before I go on the show because I’ll be a little bit more on top of it.
[Damien Blenkinsopp]: First of all, if I’m going to do some speaking or some sort of cognitive task, I’ll take a ketone. KetoCaNa is my favourite from KetoSports. Those are the original makers. I get these kind of benefits I really think it is [check 2:46:20]. I haven’t done the battery of testing like you, but I should do that because just like anecdotally I’ve heard other people talk about it as well.
[Bob Troia]: You can even compare. There’s other tropics, you can probably stack them against each other and see how your performance compared.
[Damien Blenkinsopp]: It’s the best thing I’ve taken. A lot of the nootropics, I really don’t find they impact me and often they start affecting sleep if I take them so that destroys all values right away.
[Bob Troia]: It’s not a one size fits all. I know I don’t respond to or may respond to and you’re going to be very different.
(02:46:57) [Damien Blenkinsopp]: Yeah. Everyone has got a different brain chemistry so you have to be really careful about that. So we’re going to wind down because we’ve got other stuff coming. Is there anything you haven’t spoken about that’s really cool? Or anything you want to say?
[Bob Troia]: Anything cool? I think from the quantified aspect of things, I do think there are some cool advancements happening and some of what we can measuring today. I was just inside this event and I was getting my face thermal-imaged. It’s interesting to see how technology is always getting married with Chinese medicine. So we’re really going back to these things that have been around and they seem new because you couldn’t quantify them.
So imagine getting a thermal image like Predator. That movie Predator, you look like.
[Damien Blenkinsopp]: Predator?
[Damien Blenkinsopp]: Yeah and you’re lit up so you see hot spots and cold spots. So they did my face and they could tell I had just arrived off the plane and they could see that my throat was all irritated. They saw my nasal passages were [check 2:47:43]. Then they can map the Chinese acupuncture points and actually show you right here you have some poor digestion happening just by looking at the thermal camera. Now you can actually put this to data. These are things that are quick slot. It took literally 15 seconds to do this scan. You stand in front of the thermal imaging camera, it provides the data.
I’m experimenting with other modalities that are coming from Europe or maybe the Soviet Union that they’ve used for athletes for years. It’s cool to experiment on some of these things. Things are getting exciting. It’s all about being able to learn even more about ourselves in the least intrusive ways and getting actual insights on this stuff.
So for me, I’ve definitely gone and tested lots of things, there has been lot of dead ends and things that are cool, but is the benefit worth it? There might be things where it’s a hassle and I’m not getting enough out of it.
[Damien Blenkinsopp]: A lot of it’s you do projects, you add stuff, you retest it for a while and then you eliminate, you start cutting stuff. It’s this constant process of push forward, add some things, remove more things to get to the stuff that actually is worthwhile.
[Bob Troia]: So the analogy I made with biohacking and quantified self not all biohackers would call themselves quantifiers. I’ll try 20 things and I feel amazing and I don’t really care about isolating what worked. Maybe it was only one of those things that really contributed to it, but they’re not really interested in isolating it. They’re just like I’ll just do everything. Whereas the quantification side, well instead of taking 20 things or doing 20 different things, if there are two or three I can do that I get 90% of the benefit from, I’ll do that. Because 90% of the benefit with the least effort. That’s efficiency.
[Damien Blenkinsopp]: Yeah. It’s a more [check 2:49:34].
[Bob Troia]: Yeah absolutely. In structured experiments, you always do like a ABA test .
[Damien Blenkinsopp]: It’s repeat. I really find value in the repetition. You cycle on, you cycle off, you cycle on, you cycle off, you cycle on; you do that those four times and you can have a clear signal.
[Bob Troia]: Absolutely, yeah. We’re all seeing a subject experiment so you don’t have to worry about the scientific rigor.
[Damien Blenkinsopp]: As long as it works for you, who cares? It’s like in n=1 If ultimately that’s what we’re out for. So we’re not doing science for everyone. It may be useful as a case study for someone else, but then go and do some science, but there are more important things that actually just work for us.
[Bob Troia]: Yes. I agree 100%.
(2:50:20) [Damien Blenkinsopp]: Ok so where can people find you? Just a reminder where are you most active? Where would you tell people to go?
[Bob Troia]: Sure. So Quantified Bob, you can go to quantifiedbob.com. Any social media, Instagram, Twitter, Facebook, Quantified Bob. You can email me, Bob@quantifiedbob,com. If you’re ever in New York City or you want to start playing around with some cool tools and training and recovery tools, if you go to Optml O-P-T-M-L optml.co, you can see some of the things that I’m doing up there with the oxygen training and recovery tools and that will be built out over the next month or so.
[Damien Blenkinsopp]: Excellent. Thanks very much for your time and I’m sure I’ll be seeing you at another event soon.
[Bob Troia]: Yeah, it has been great. It has been so great hanging out with you and reconnecting and looking forward to the rest of the event.
[Damien Blenkinsopp]: Yeah, me too.
[Bob Troia]: Thanks.
[Damien Blenkinsopp]: Turning you guys off. See you later.
Hey there! Congratulations on getting to the end of a Quantified Body marathon episode. I don’t know about you, but I had a lot of questions coming out of this conference and on the discussions I had. It was a good introduction to get the lay of the land, but I have a lot of questions particularly before I would consider actually experimenting with any of these tools that were discussed.
So here are some of my first questions. I’m bringing them out there so that if you have any thoughts yourself, you can perhaps add your comments or your questions to the blog and we can have a bit of a discussion around this because I think there is a lot of uncertainty. There is a lot of different things to tackle and topics to explore in this area and it’s really for me, this is like a first episode of many future episodes. This is an important topic to me and I think an important topic to everyone and it’s going to be more and more interesting in the next years.
So here are some of my questions that I have after this episode. The first area is really understanding the risk profile of some of these tools. To make sure that there is no huge downside basically to the use of any of these tools that we are completely unaware of or some blind spots there. In particular, there are a couple of ones that I’m interested in trying to understand that risk profile better.
So that would be senolytics is number one. My questions are: How can we evaluate the risk profile of some of these different senolytics? Who should take them, who should not? At what age should you be? At what age does the upside become more useful than the potential downside? What is the track record in the use of some of these? Do we really understand them? Even the ones like some of the antibiotics or the chemo-based drugs that have been used for a while, potentially we don’t understand all of the long-term effects of these.
On my journey in the Quantified Body, I’ve learnt that we are still learning a lot about the body and we are learning our ability to quantify and get data on aspects of our biology is still very limited. I expect this area to be transformed in the next 50 years with just the amount of data and understanding that we can actually process. So for now, I consider that most of our biology is not being tracked. We don’t have data on it and it’s just a big black hole that we don’t know anything about.
My concern for these things are is there something going on which could present some long-term damage that we’re not aware of. How can we ensure that we are preferentially killing just senescent cells and not doing some other kind of damage? So that’s the topic I’m interested in understanding more before I potentially experiment with this myself.
The second one would be in the area of young plasma. I think this is very, very similar in my concerns. My main concern here is with blood transfusions in general. If you’re not in a critical state so if you haven’t just had a car accident and it’s really life or death, you need a blood transfusion to survive, then what is the risk profile of having a blood transfusion?
I believe that we aren’t able to screen for all of the pathogens in the blood currently. If you look at some of the more advanced labs which are trying to look into this area like Aperiomics which I discussed in the last episode, episode 51 for microbiome, Aperiomics does analysis against its database of pathogens which it’s still building for all types of samples; urine, blood and stool. They’re finding things that they didn’t expect.
So I do believe that the blood samples we have today, they are screened for some of the most important infections we know of such as HIV. But there are potentially many that we are not aware of that could lead to chronic disease later in life or chronic issues in the long-term and we’re just not aware of them.
So I feel like there is a risk profile there to establish on blood. If you’re going to have a transfusion of younger blood, then how do you ascertain that there’s nothing in there that could present some issues in the longer term and thus negate those young healing benefits from young blood? So that’s understanding the risk profile better in particular separating out any larger downsides rather that we may be exposing ourselves to and are unaware of.
The second area is really trying to understand the benefits and the upside of making an effort investing money in these treatments or these tools. So really understanding it area-wise. Is it worth our time?
The two which would fit more into that category now I think are Rapamycin because this is available now. You can get this. What kind of protocol could you put in place? What kind of experiment could you do? What biomarkers could you be testing in order to understand over a year, over two years, does this have any benefit to you? Is it worthwhile from a cost and effort perspective? Or potentially some of the side effect downsides? Also we have to take those into account. So would it be worth it to you?
Then the other one is NAD which has received a lot of press over the last couple of years because there has been nicotinamide riboside (NR) which have been on the market and popularized a lot by the company Elysium Health in the form of its supplement basis which you may have heard of. But how worthwhile is NAD supplementation really? There is a little bit of conflict around this in terms of the scientific discussion around it. How interesting is it? How beneficial is it given the cost of these supplements currently?
So someone who I did meet at the conference and I interviewed for the video live, but isn’t in this audio episode is Maria Entregus. Her grandson has worked with SENS Research Foundation a long time and has also just brought out a test for NAD levels which is a biomarker to help establish if taking nicotinamide riboside is having an impact on your NAD+ levels.
You could get a baseline an you could get some other tests down the line just to see if the value of that has actually changed. The name of that test is Life Bridge Test. By the time this episode is out, that could be out so that could be something worth looking at. Getting a baseline and then tracking that if you’re going to invest in taking NAD and trying to raise your NAD+ levels.
Those are some of the questions I have and some of the bigger questions I’m going to spend a little bit of time looking more into to understand if it’s worth doing any of these in the shorter term. I’ll be going to some other conferences in the near future. One in Berlin in March 2019 is “Undoing Aging” which I’m going to.
There are some others, some events, more events that are taking place so you can expect some more updates on these technologies and potentially some self-experiments if I decide one of them has a reasonable risk profile and I can track some of the upside benefits there.
So I’d love to hear your thoughts on these questions if you have any or if you have ideas, any clear ideas on them or references of course. We like references. Or if you have your own questions about these, please post them in the comments of this episode on the blog. I’d love to hear from you. So you can do that by going to thequantifiedbody.net and then pick out the episode there and comment on it. That’s it for me for this episode. I’ll talk to you again soon in Episode 53.